
- List of Photomicroscopy Plates by Type -
Irregular Forms:
Gravel (Plates 1.1 - 1.5)
Columnar Forms:
Needles (Plates 2.1 - 2.5)
Capped Columns & Hexagonal Plates (plates 3.1 - 3.11)
Stellar Forms:
Stellar Plates (Plates 4.1 - 4.12)
Stellar Dendrites
(Plates 5.1 - 5.11)
(Plates 5.12 - 5.23)
(Plates 5.24 - 5.34)
(Plates 5.35 - 5.46)
(Plates 5.47 - 5.57)
Twelve-sided Crystals (plates 6.1 - 6.11)

Irregular Forms
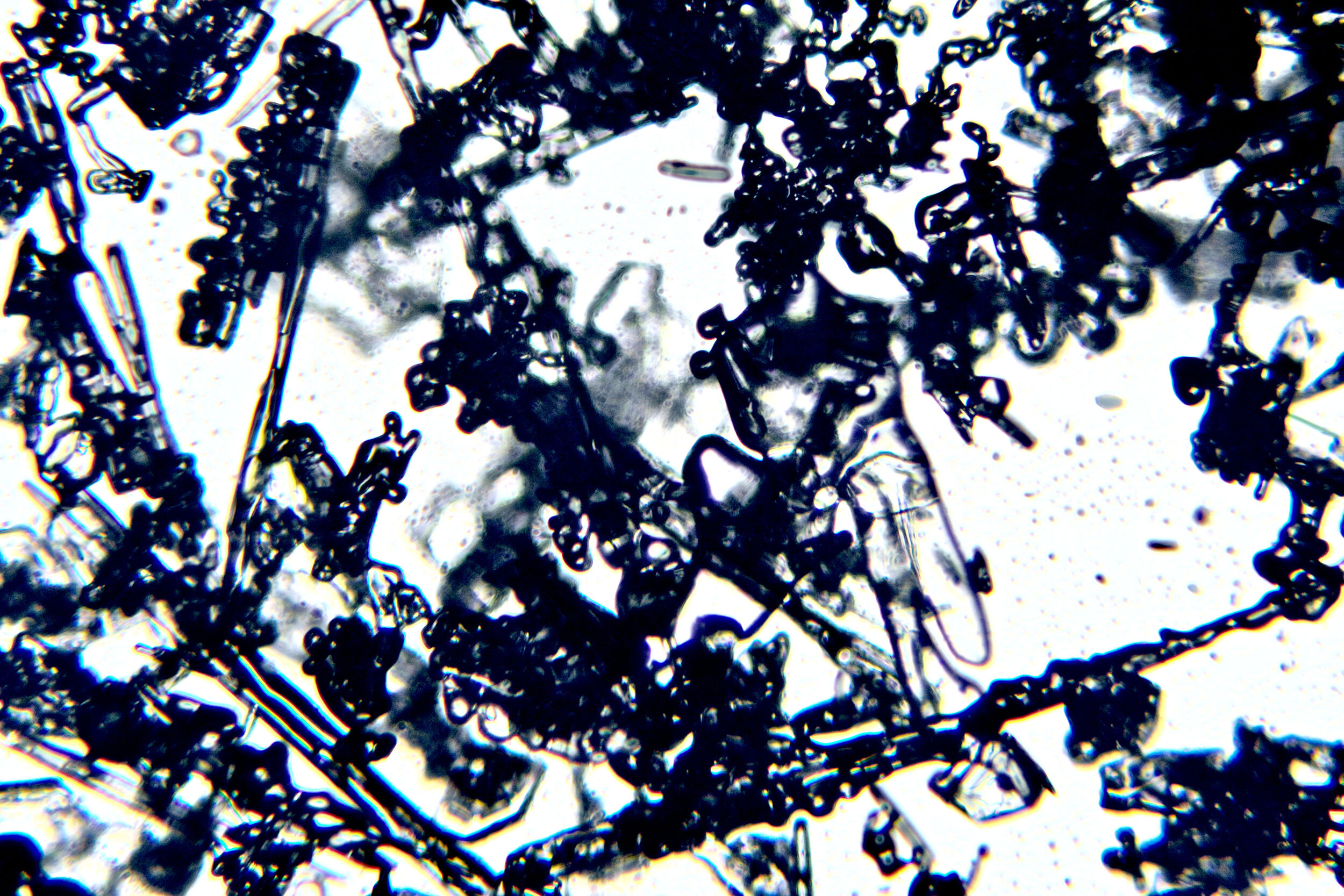
Most of the snow that falls is gravel
When we think of snow, we think of the delicate six-armed crystals that are depicted in winter decorations. Actually, the vast majority of snow falls in the form of irregularly shaped crystals that are referred to as gravel. These crystals exist individually and in clumps of varied sizes.
PLATES 1.1 - 1.5




Process Notes:
The micrographs were taken with a Nikon D7100 camera mounted on a Nikon Diaphot inverted microscope, using 2X, 2.5X, and 4X objectives.

Columnar Forms

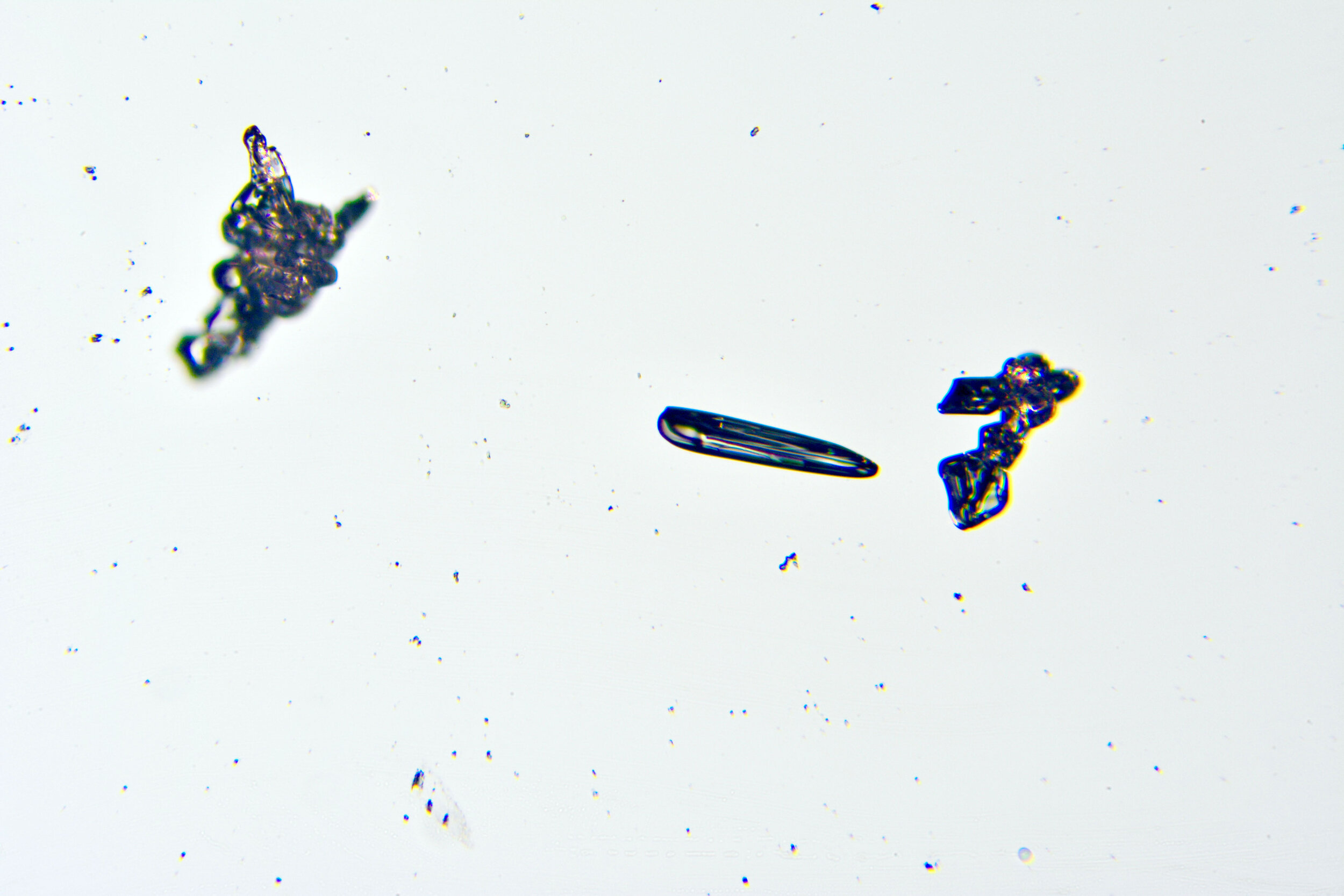
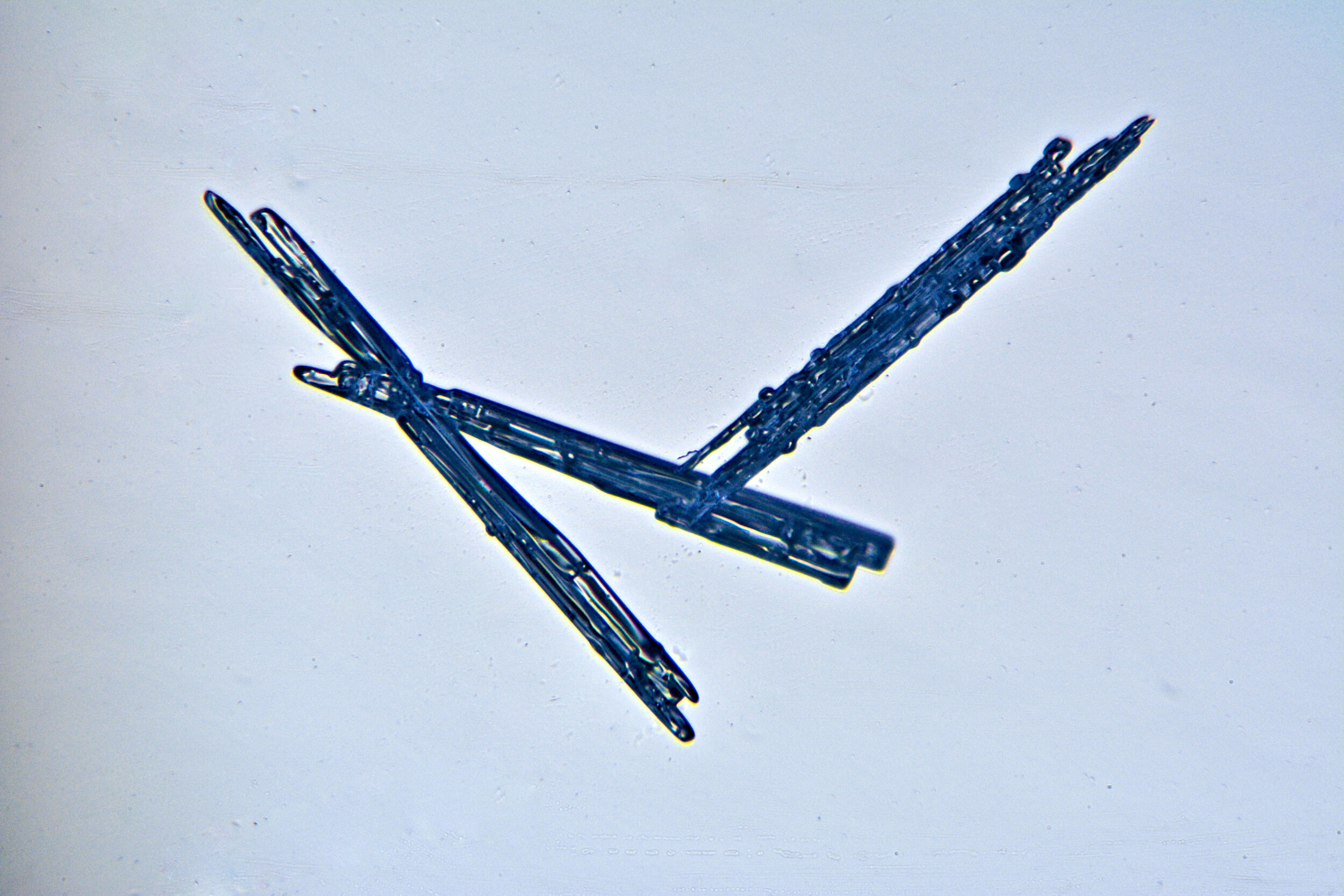
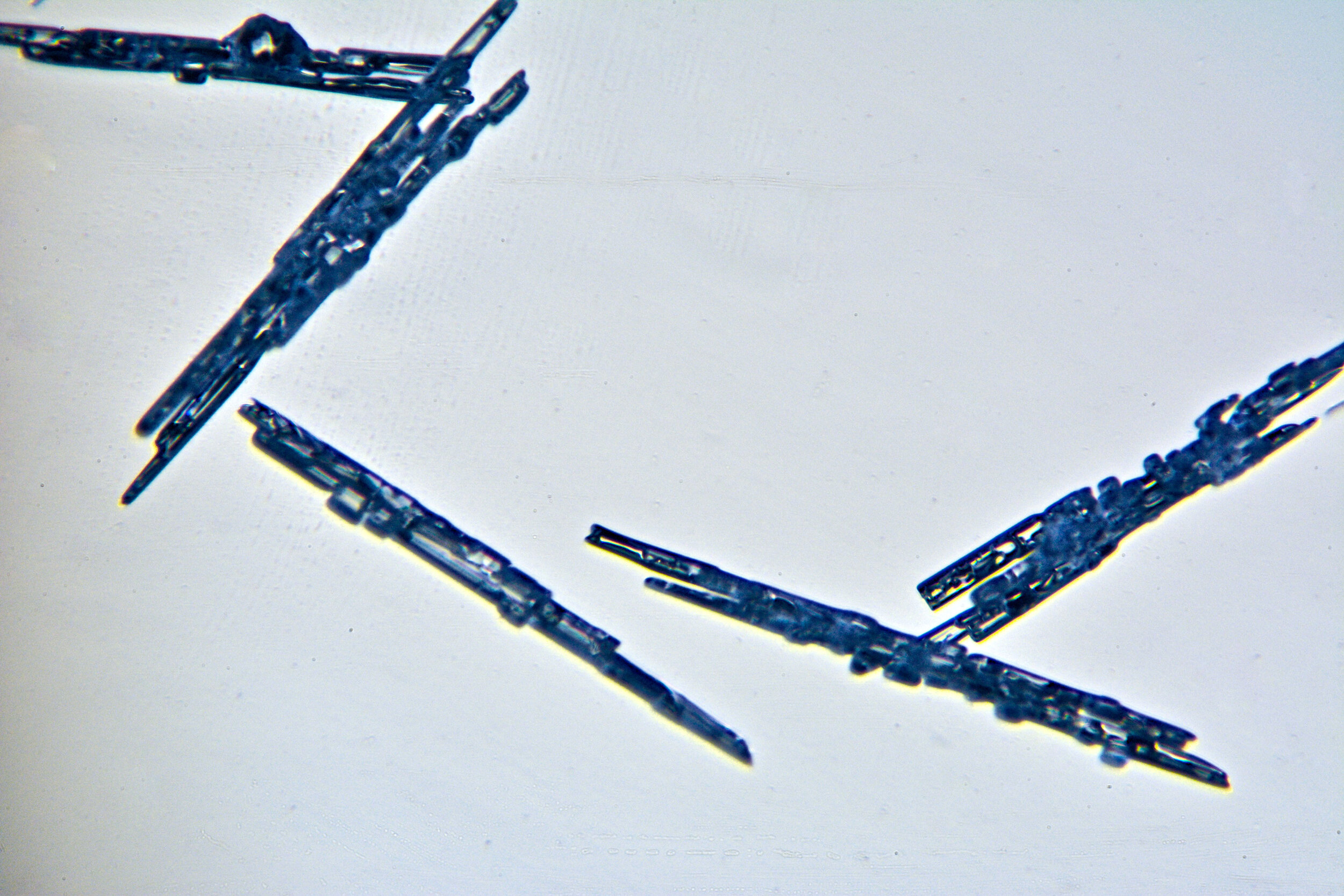
Needles
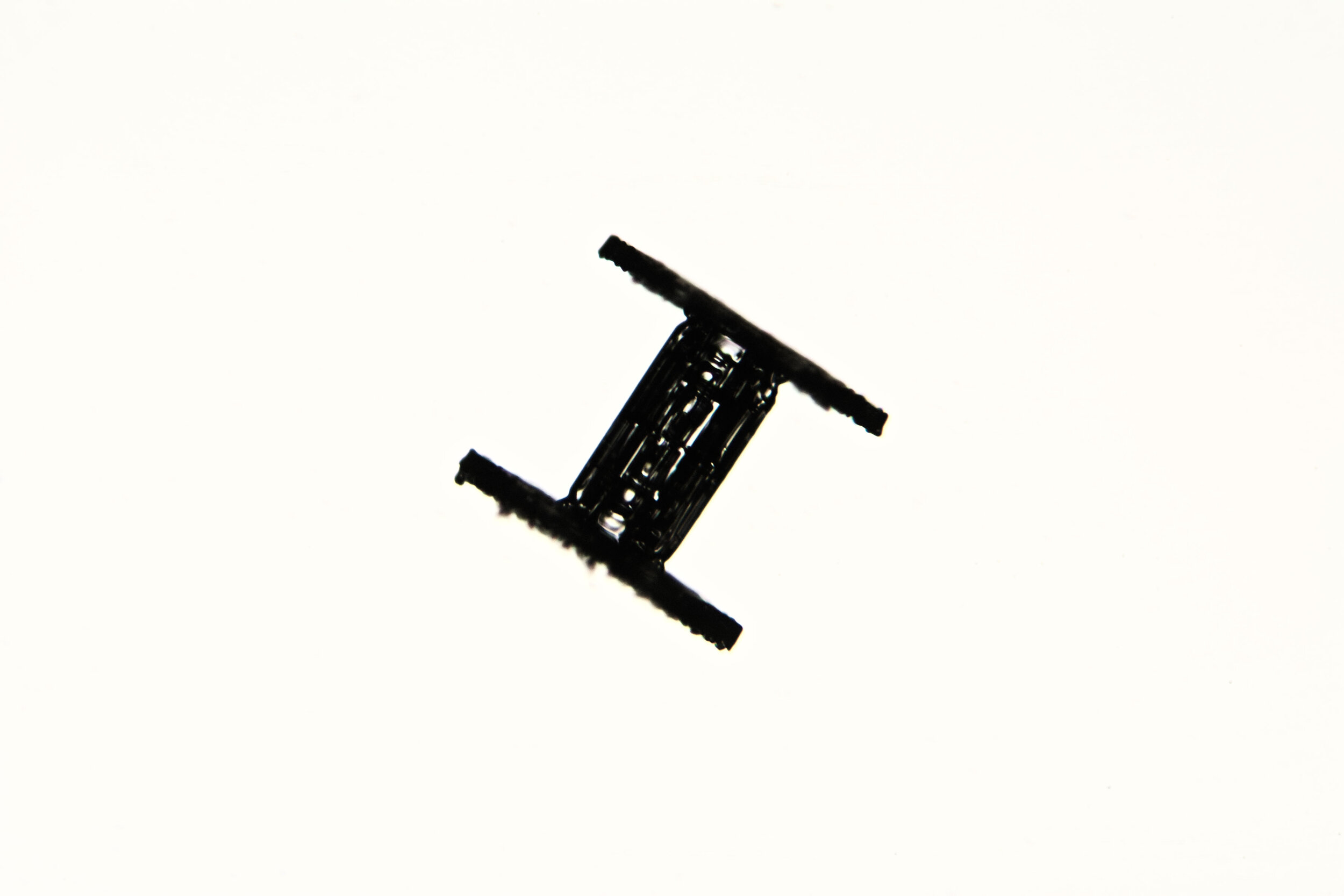
Under certain conditions, with temperatures in the low 20s Fahrenheit (-6 °C), narrow, tapered, needle-like hexagonal crystals form. These are relatively common, occurring by themselves or amongst other types of crystals. They are often found amongst the irregular crystals referred to as gravel.
Plates 2.1 - 2.5

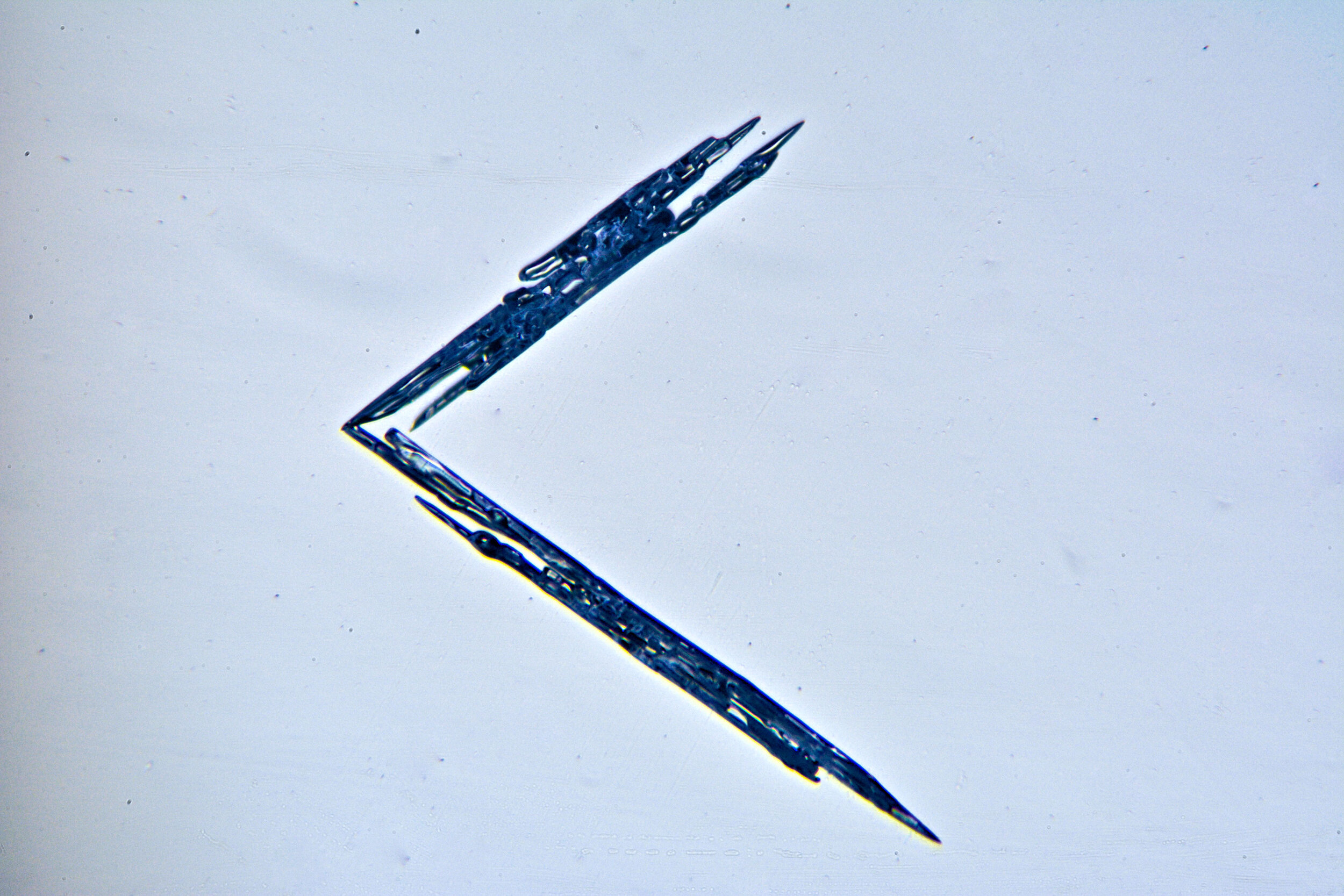
PROCESS NOTES:
The images have white backgrounds because the crystals are being transilluminated by the microscope’s light source.

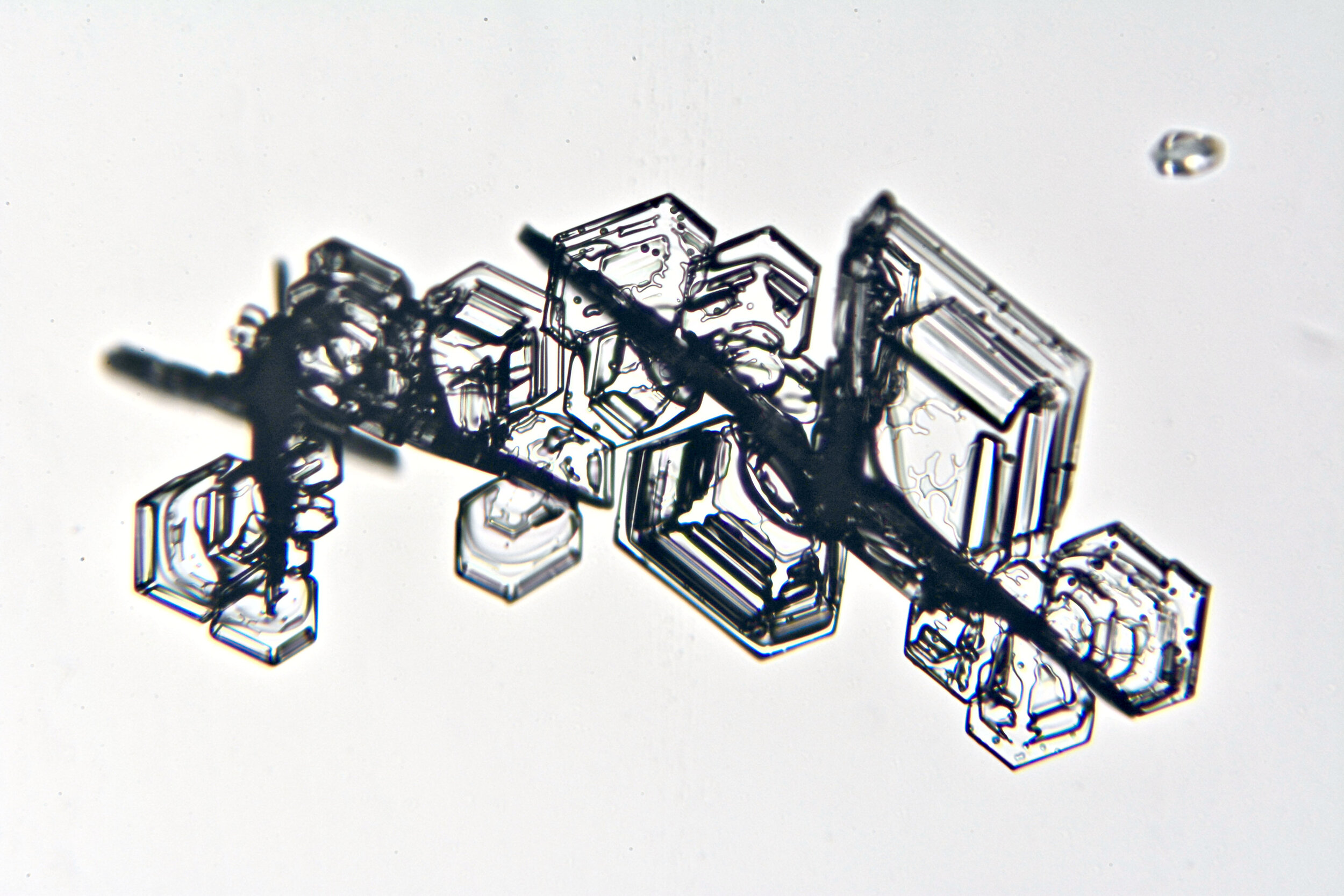
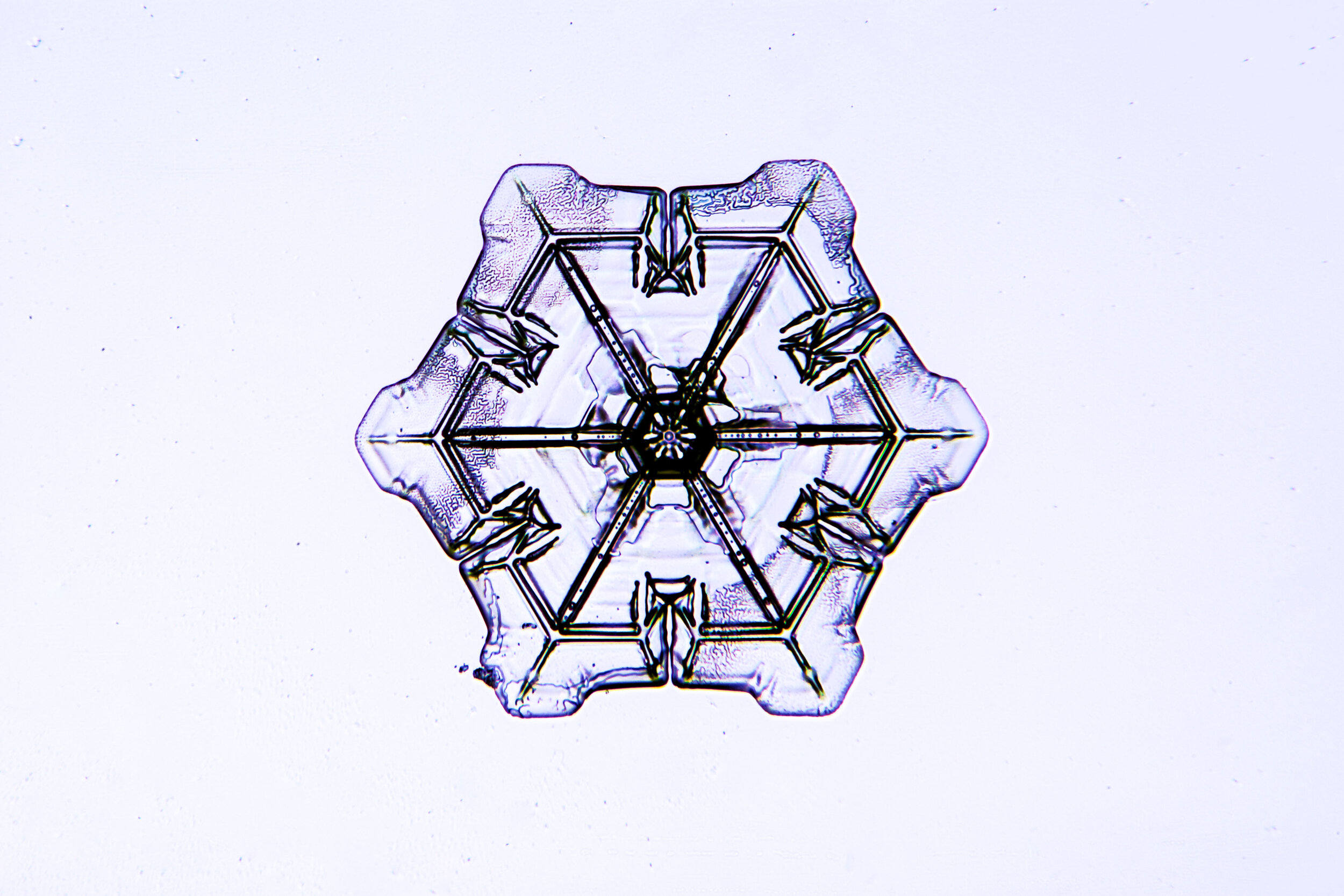
Snow crystals begin as hexagons
Crystals are solid structures formed by the highly ordered packing together of atoms or molecules. In the case of snow crystals, the most efficient formation of the water molecules, consisting of two hydrogen and one oxygen atoms, is a hexagonal structure.
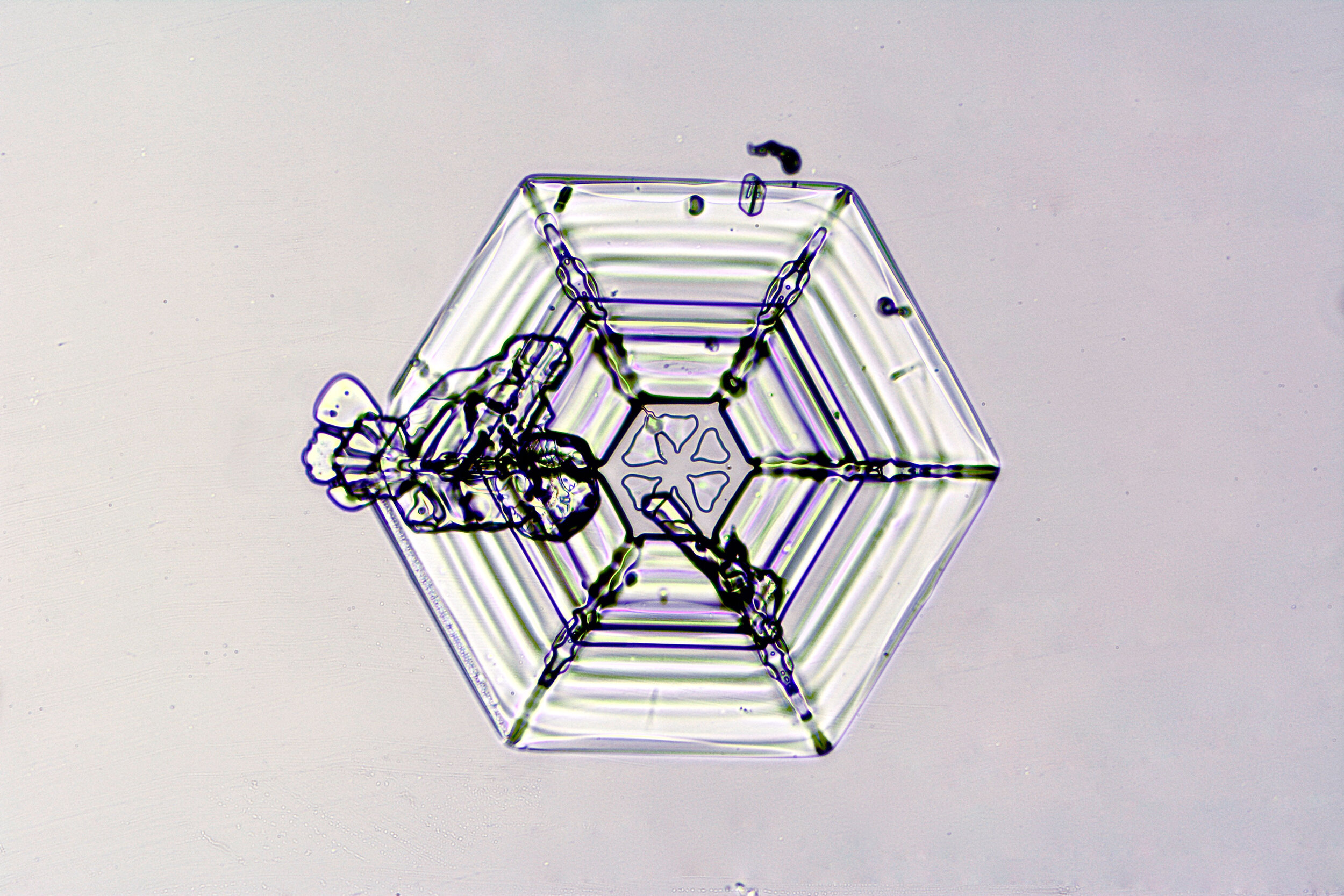
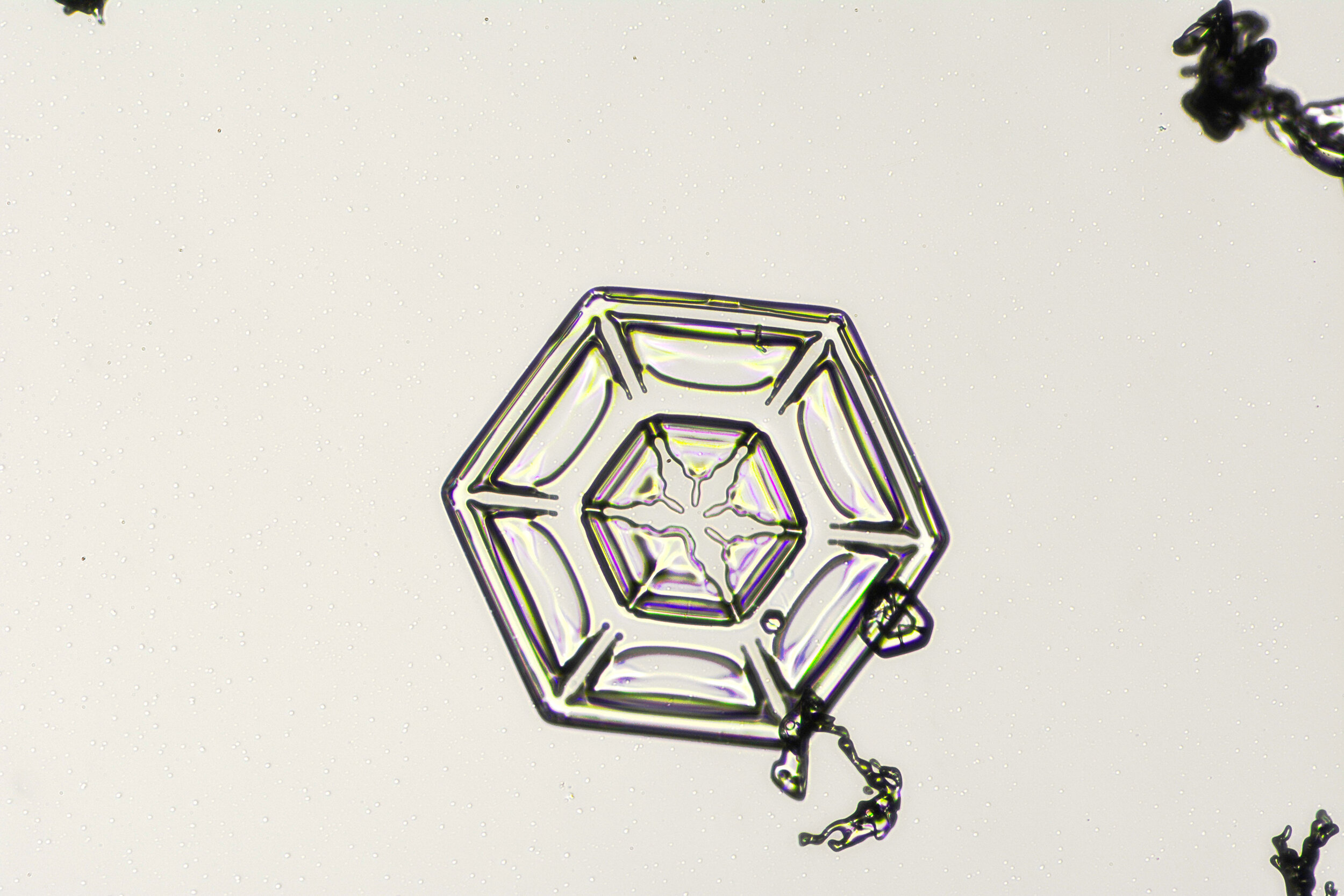
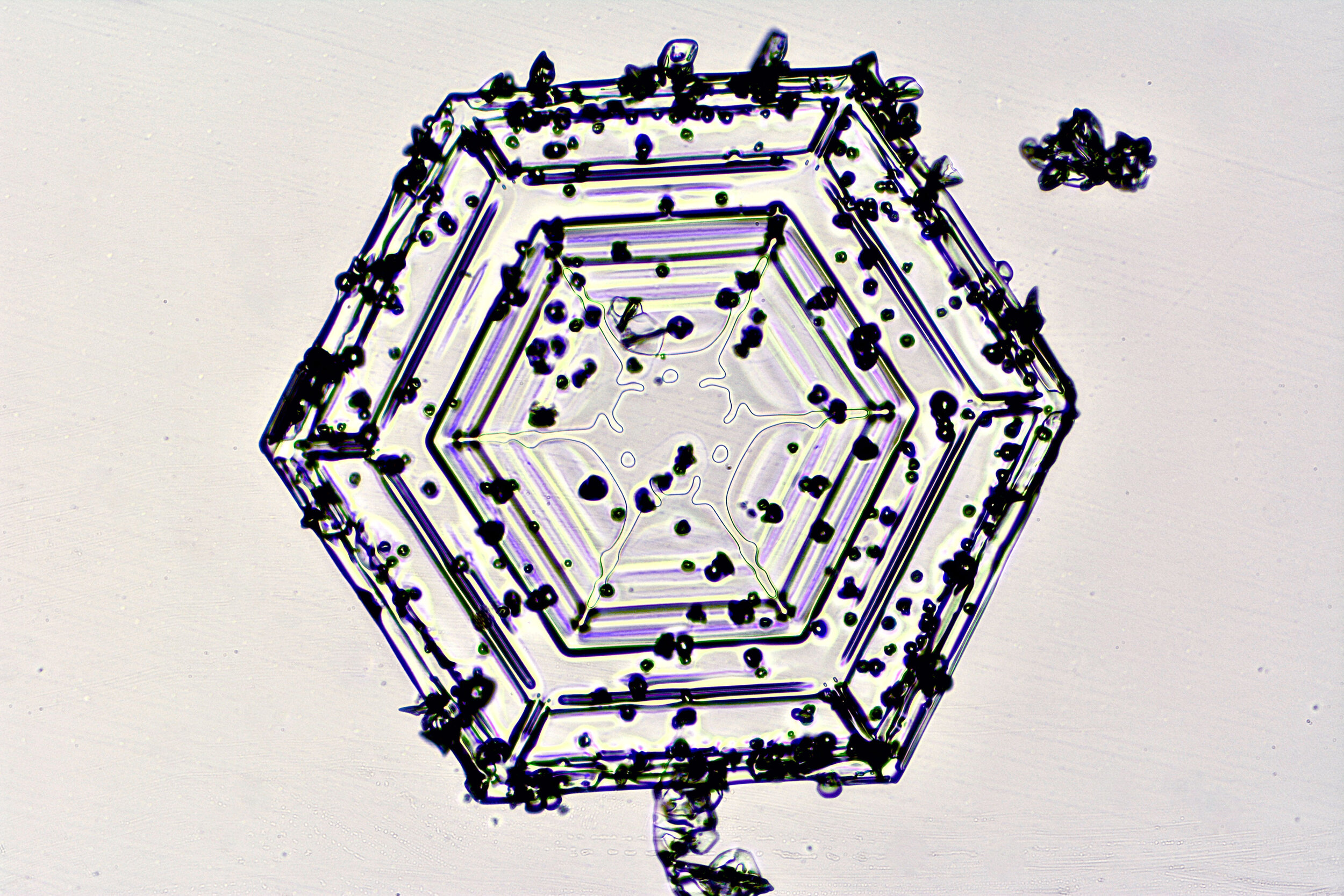
These hexagonal crystals can develop as flat plates, or in the form of columns, which sometimes have hexagonal caps at either end. The plates grow as more water molecules accumulate, and ultimately, branches extend from the six corners, and secondary and tertiary branches also form.
Plates 3.1 - 3.11

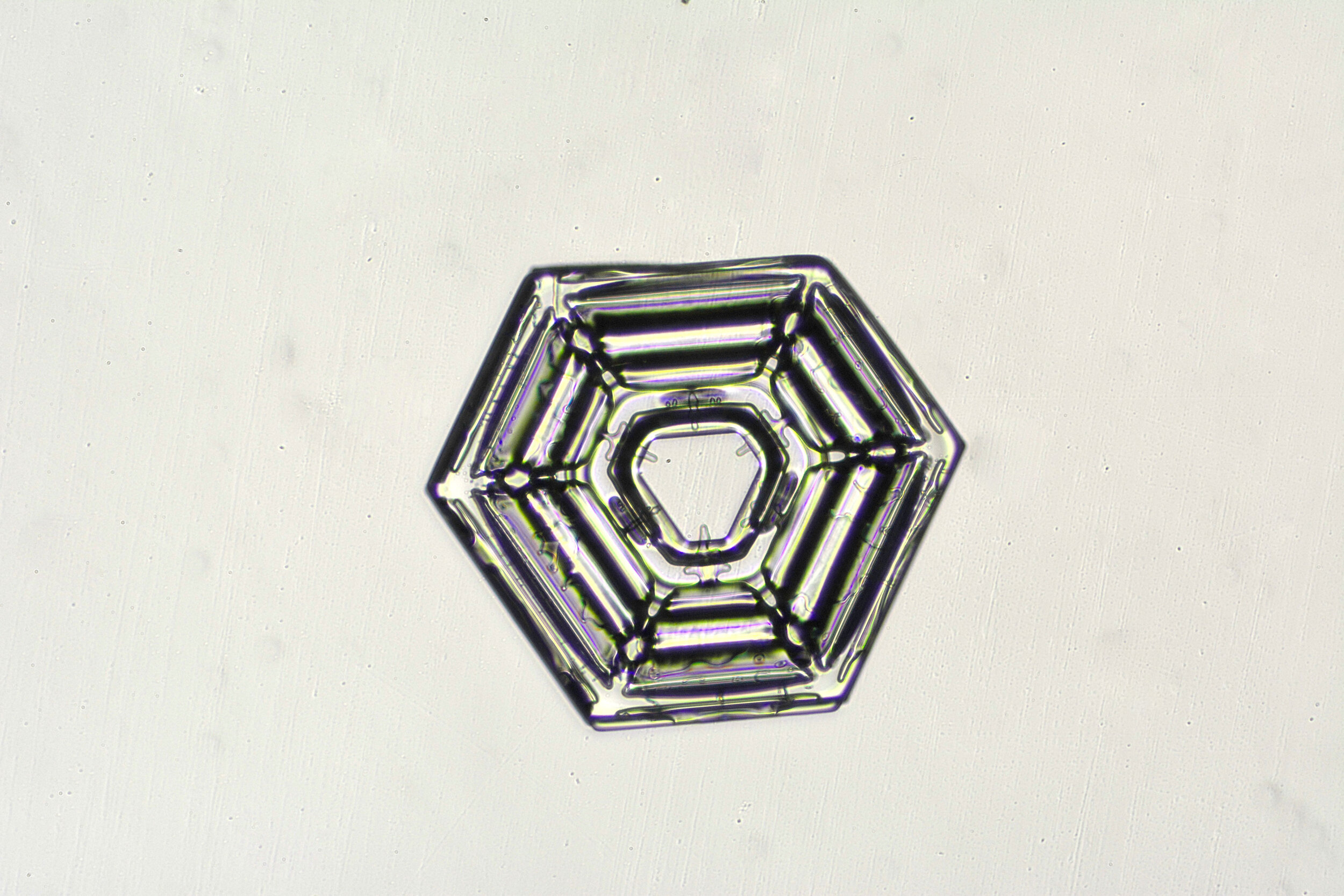
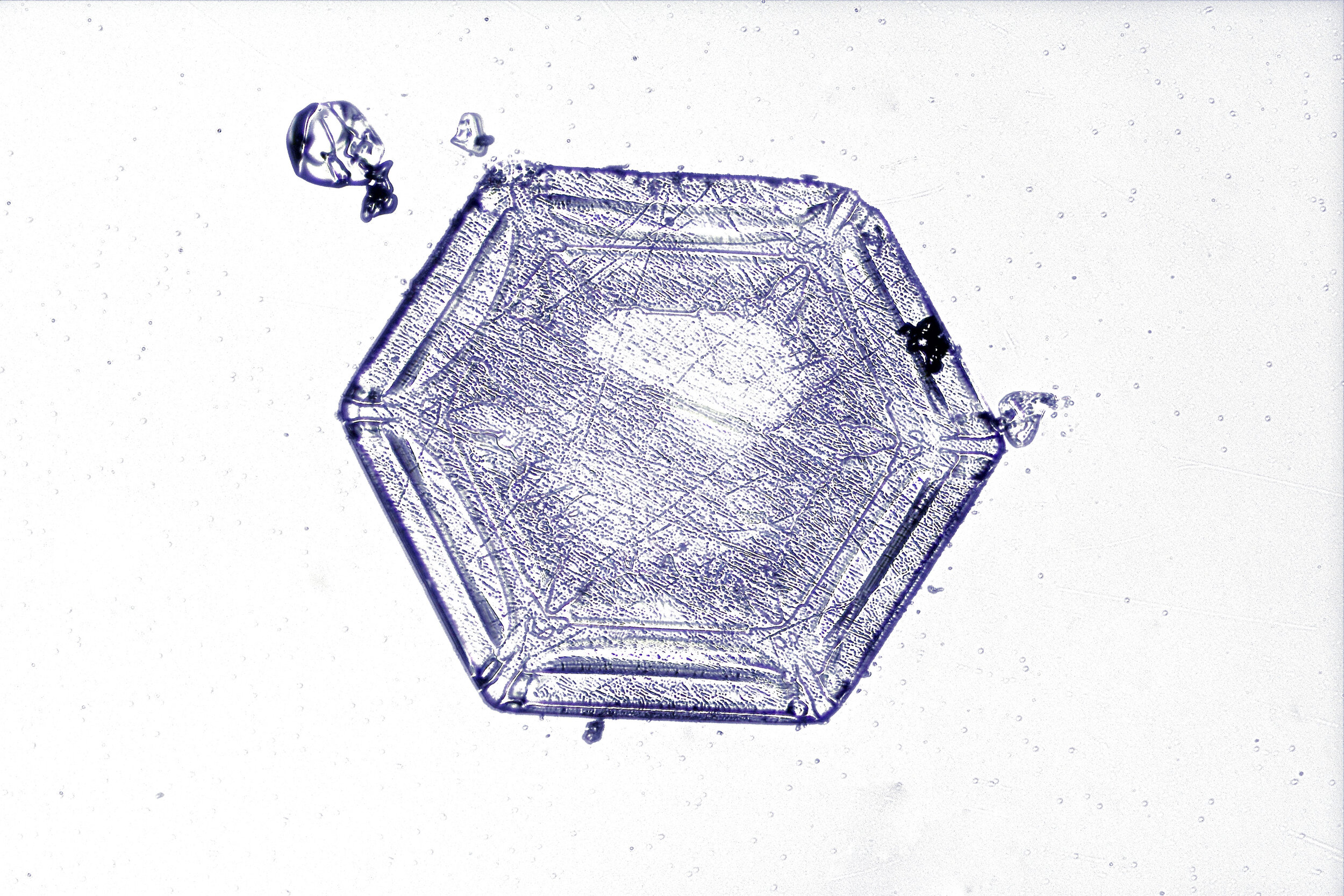

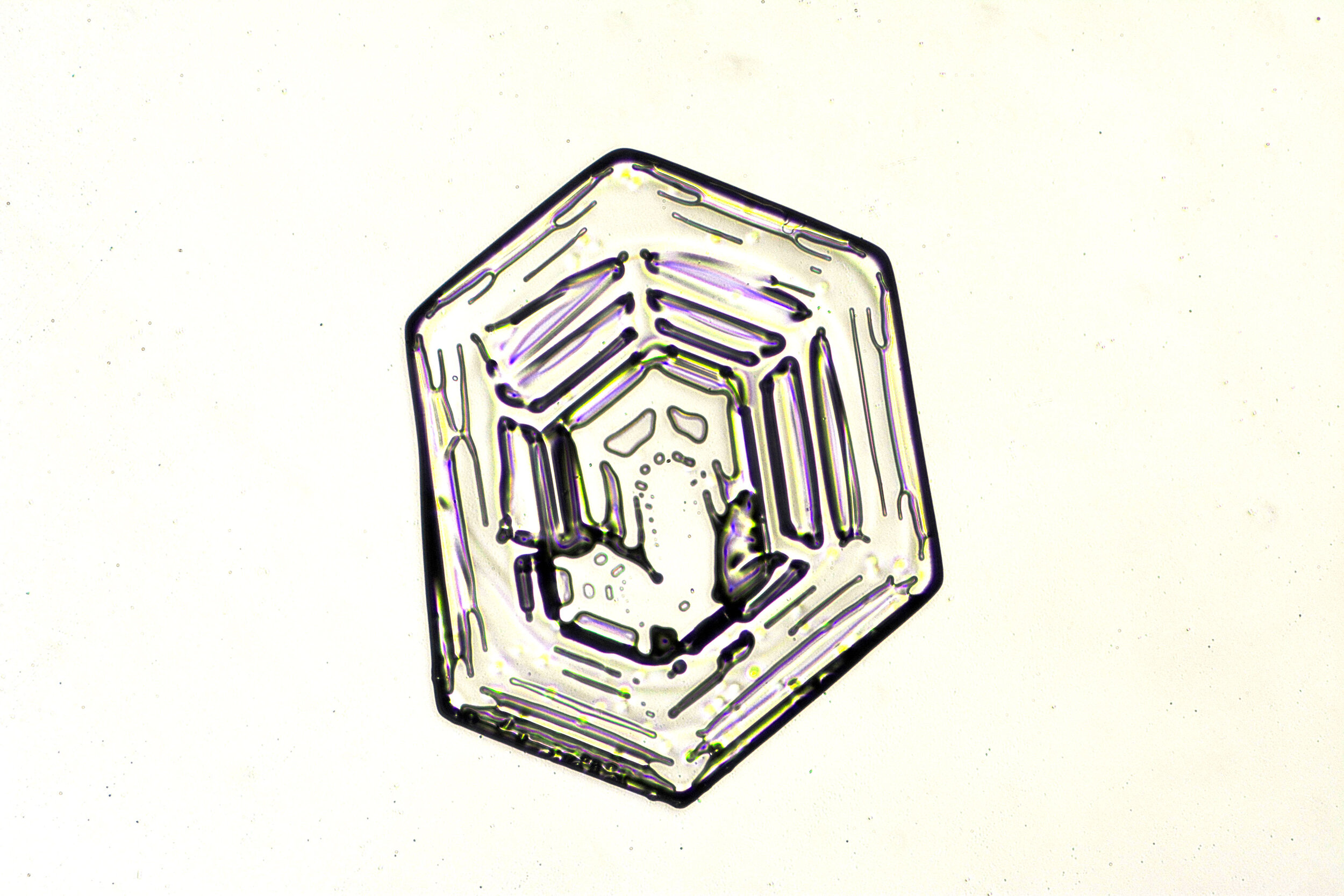
Process Notes:
Wilson “Snowflake” Bentley used a similar photomicroscopy technique, with transilluminated crystals, but he presented his photographs as negative images, possibly because people are used to seeing white snowflakes.

STELLAR FORMS

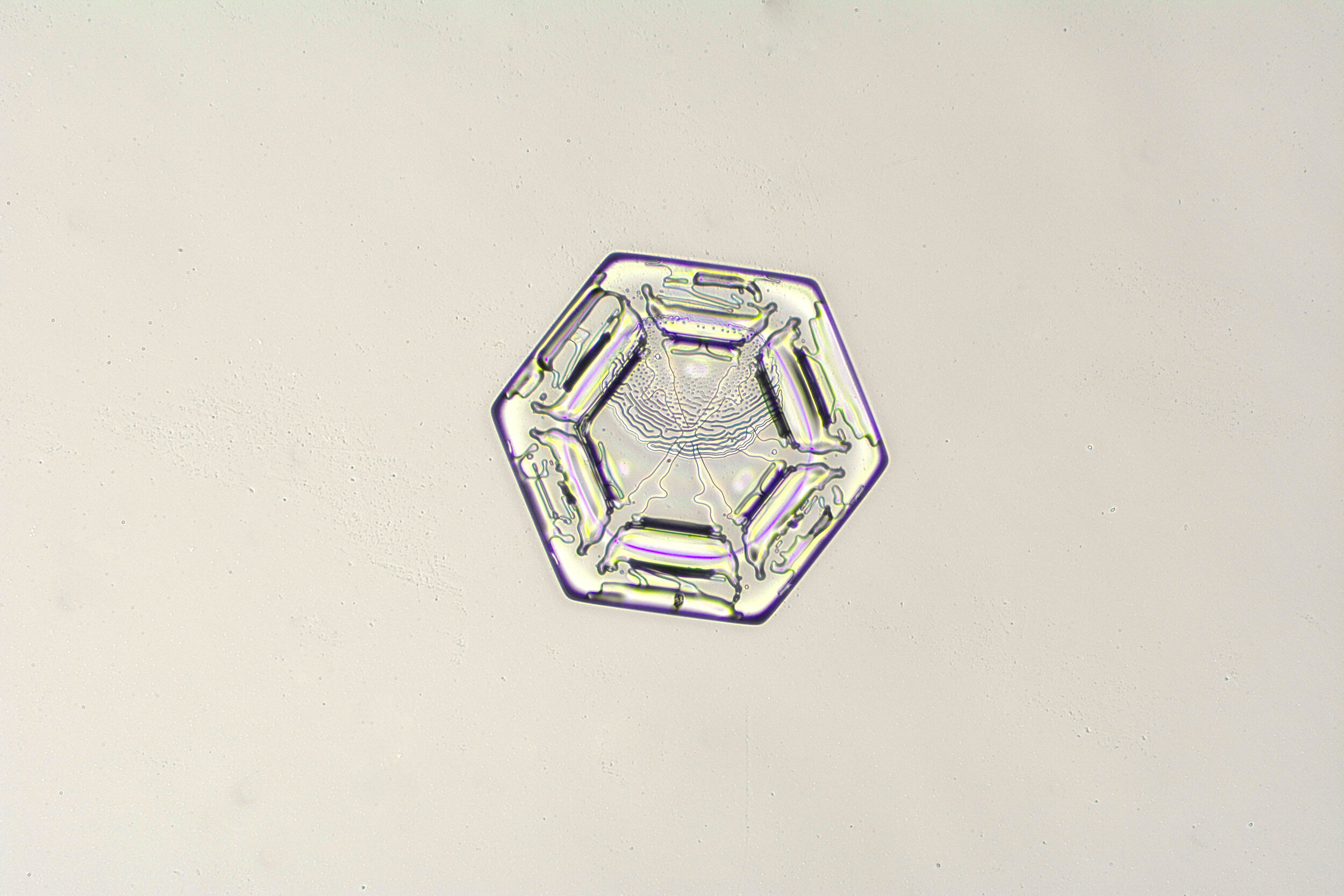
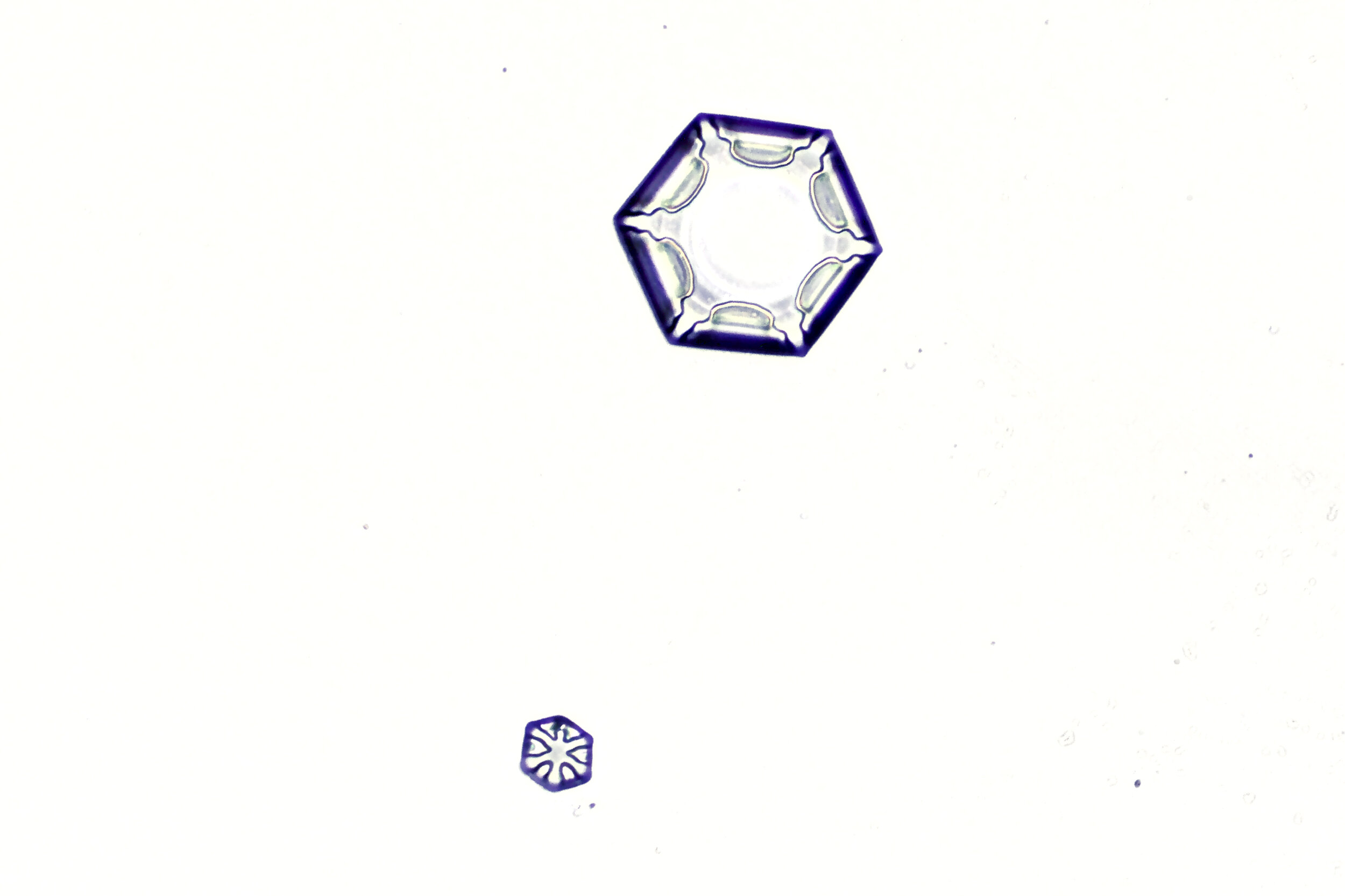
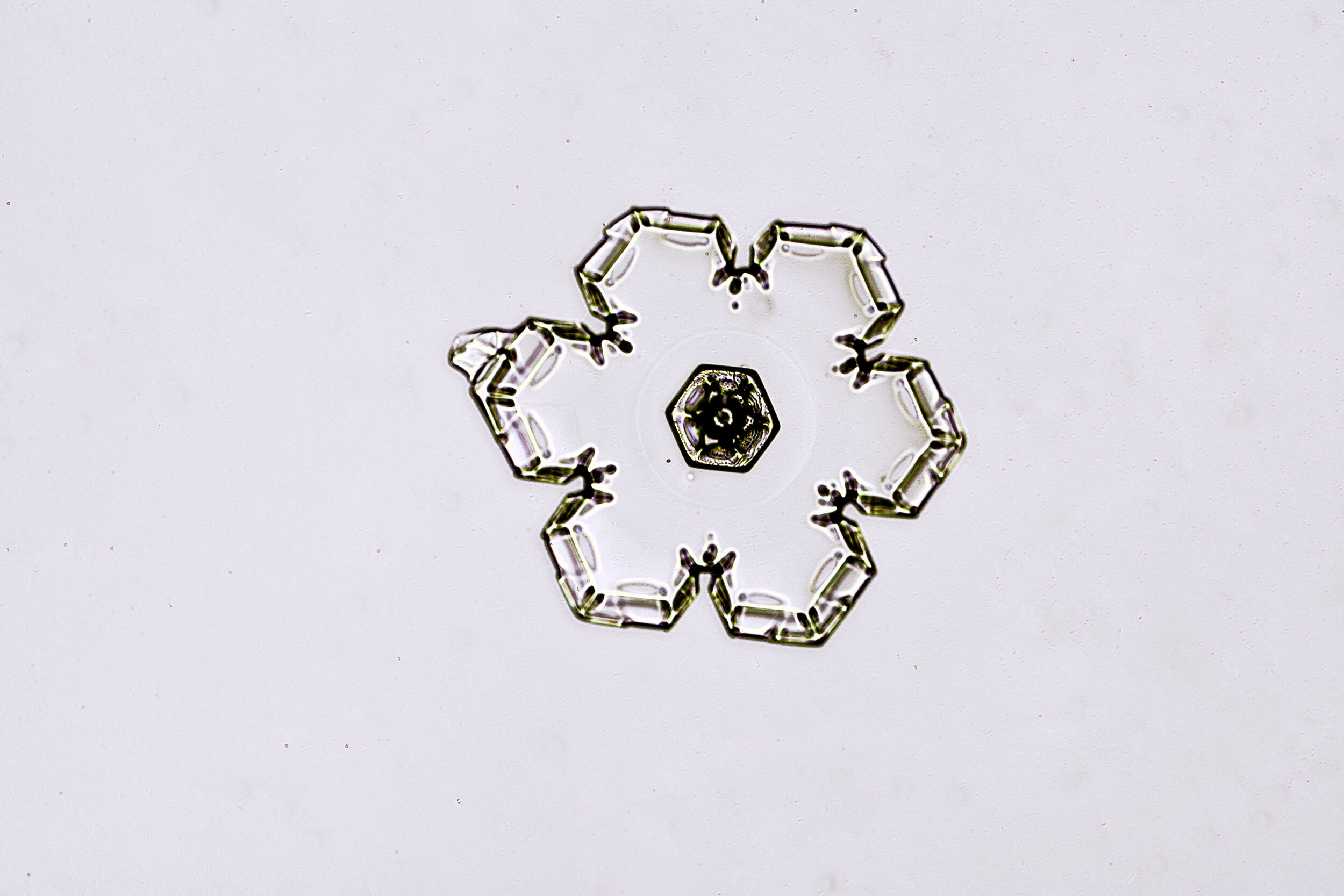
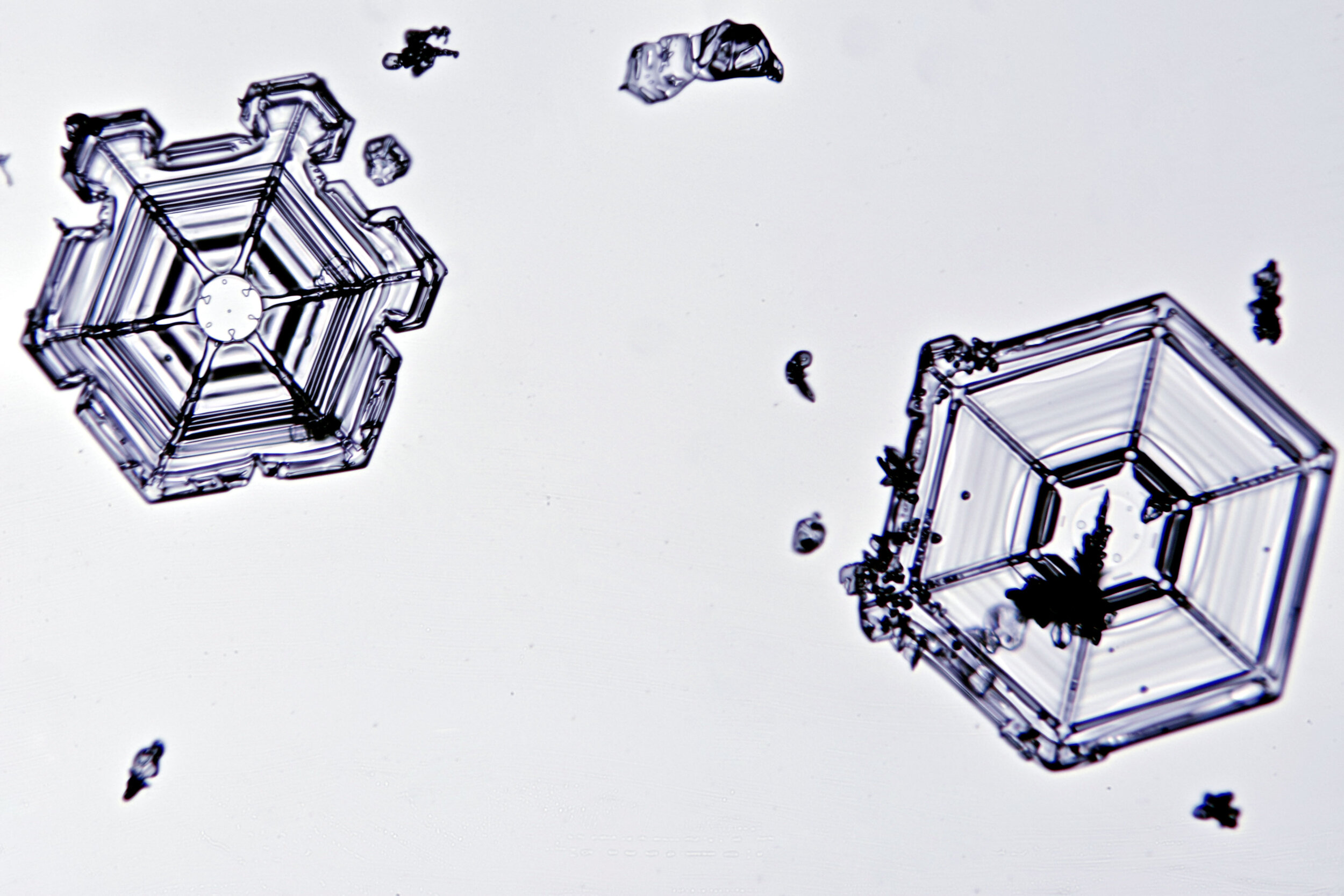
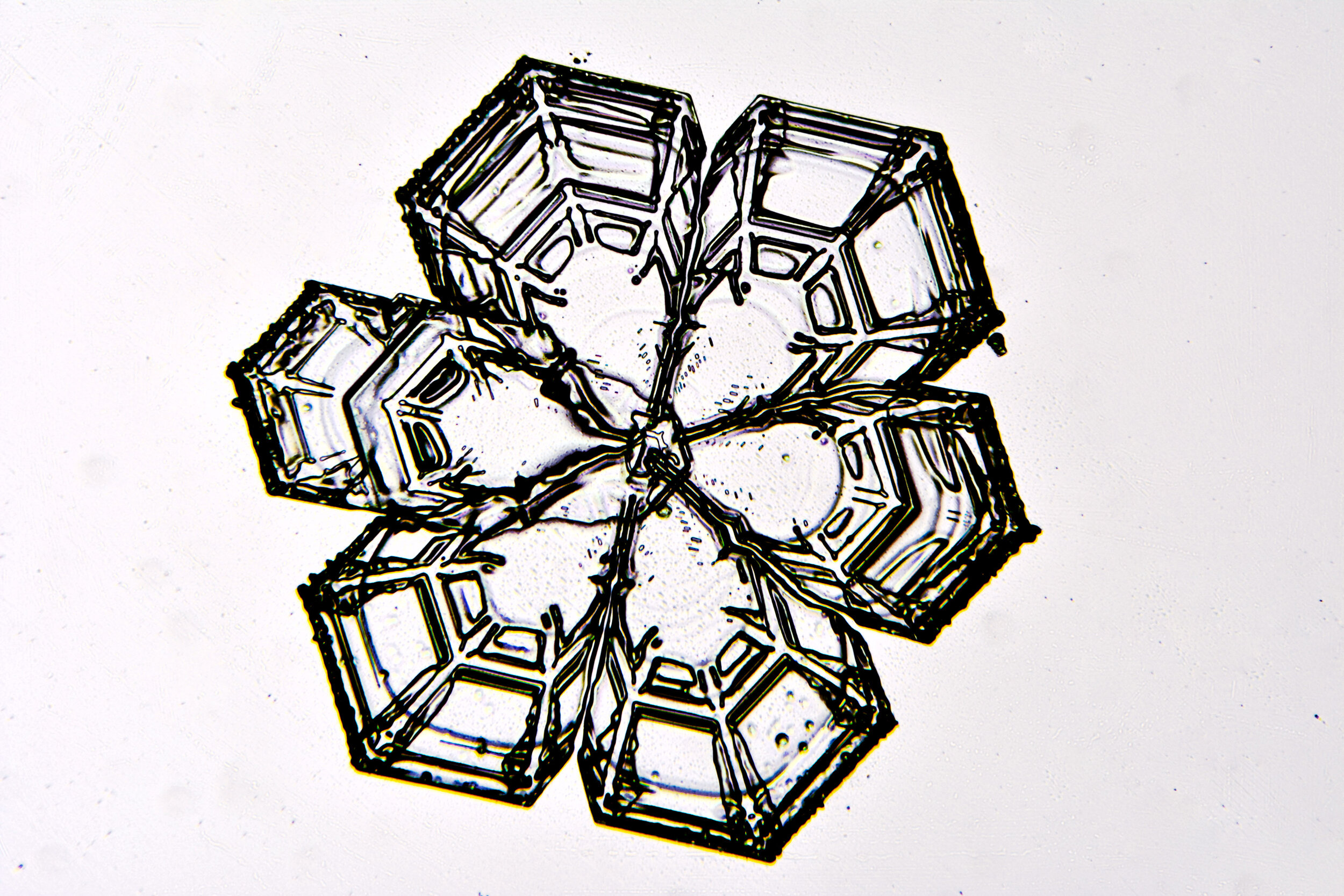
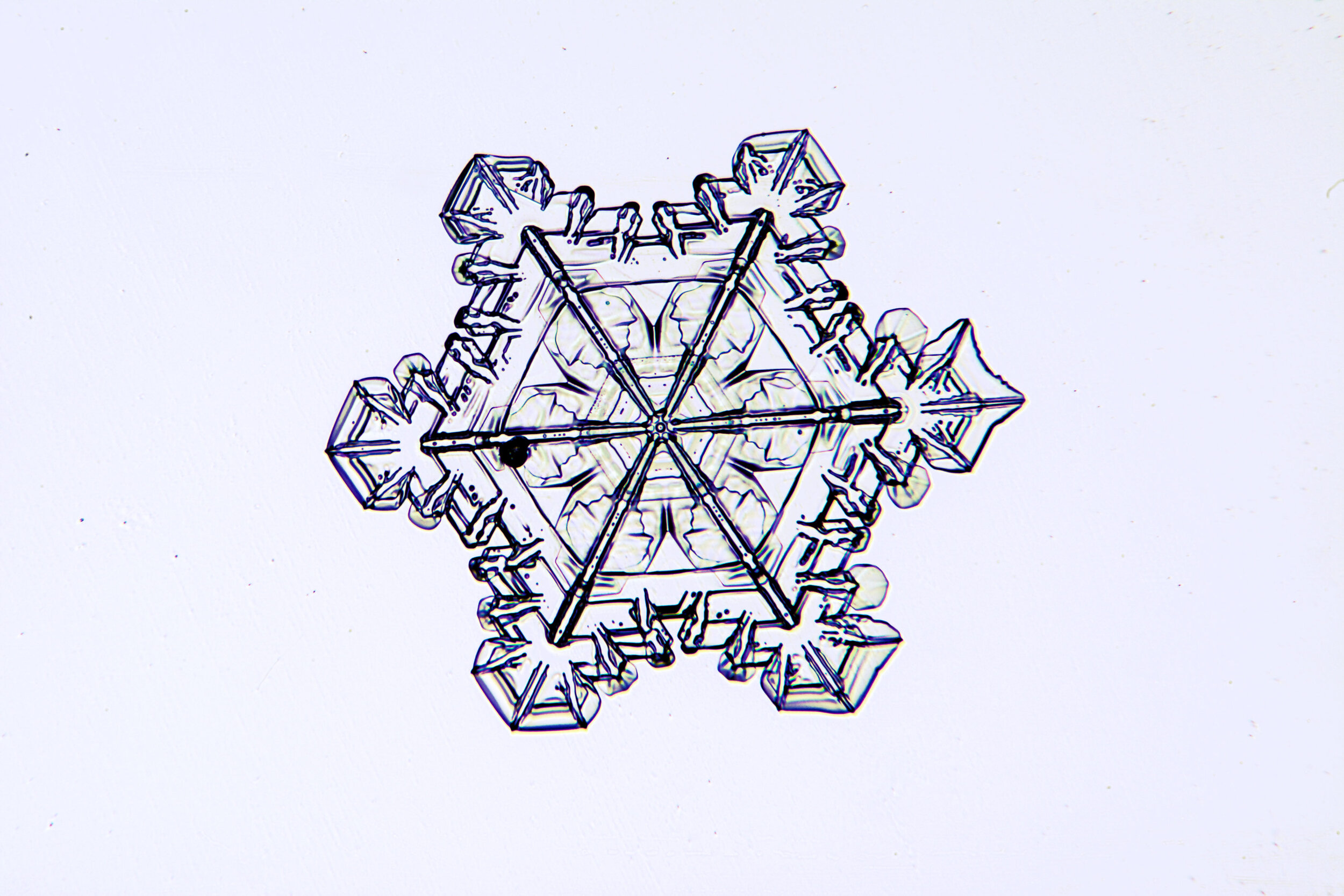
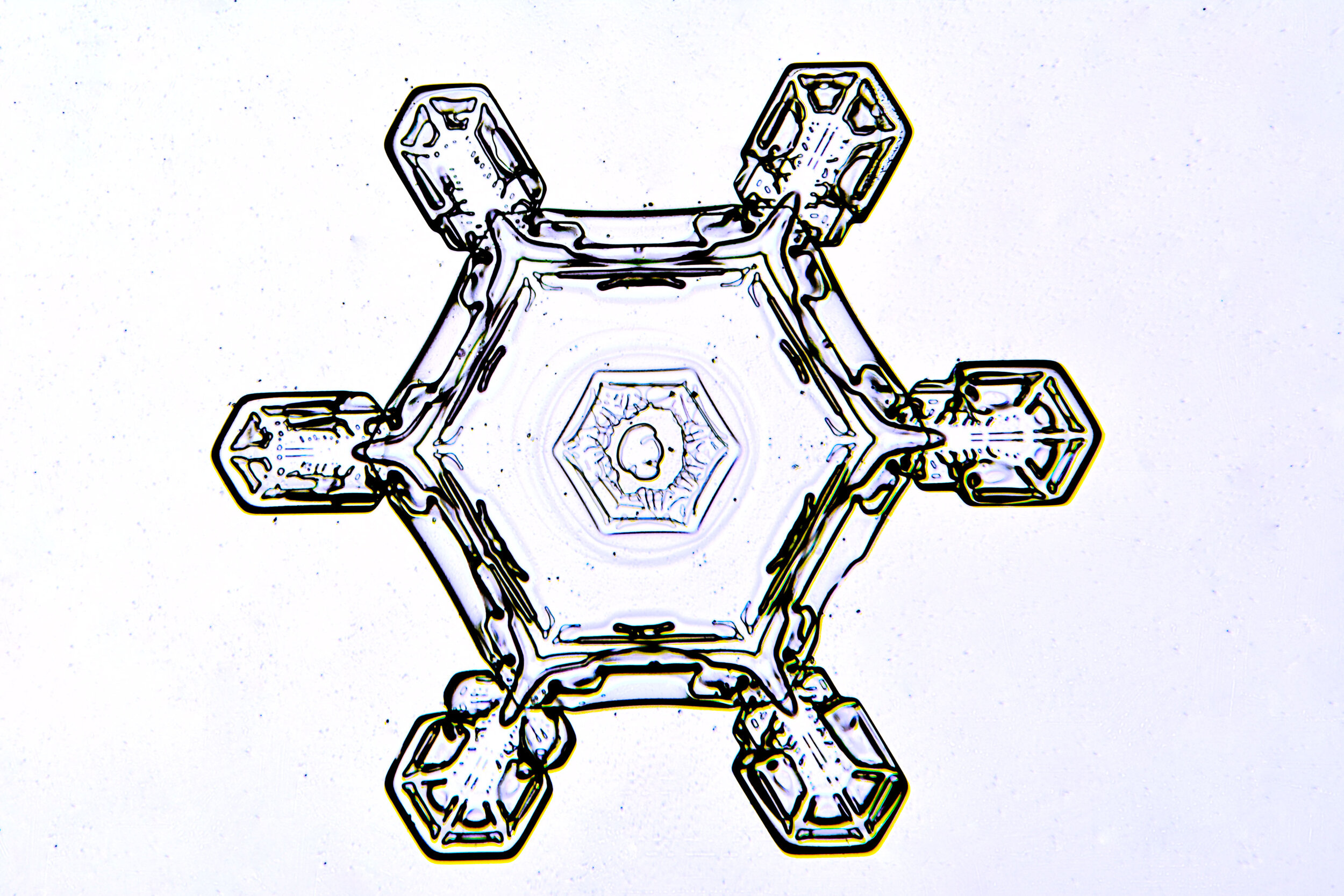
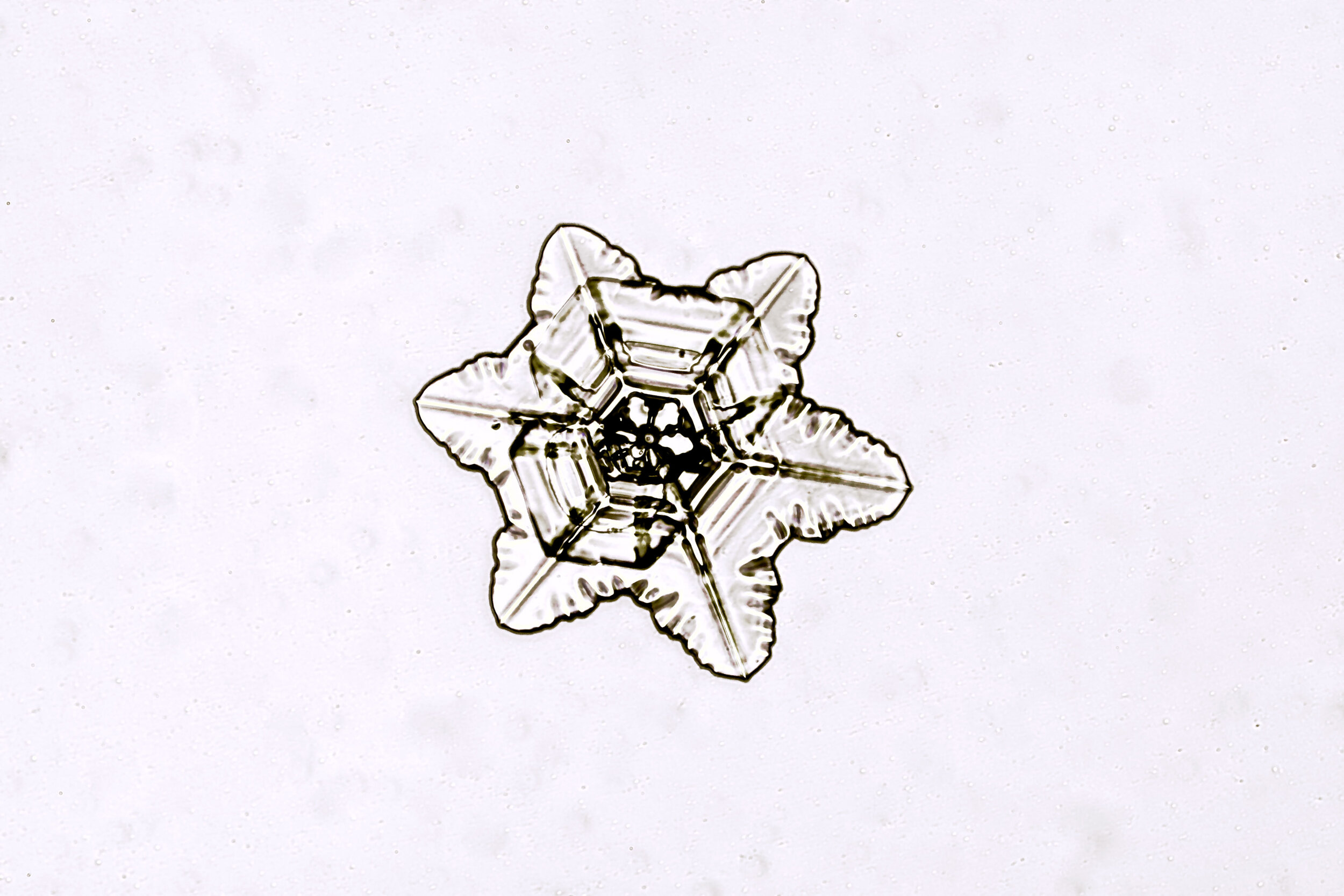
Stellar Plates are hexagonal plates with buds on the corners
When the temperature is cold enough, but not too cold, and the humidity is moderately high, water molecules accumulate on the corners of the hexagonal plates and branches emerge. Plates with short branches are called Stellar Plates.
Plates 4.1 - 4.12
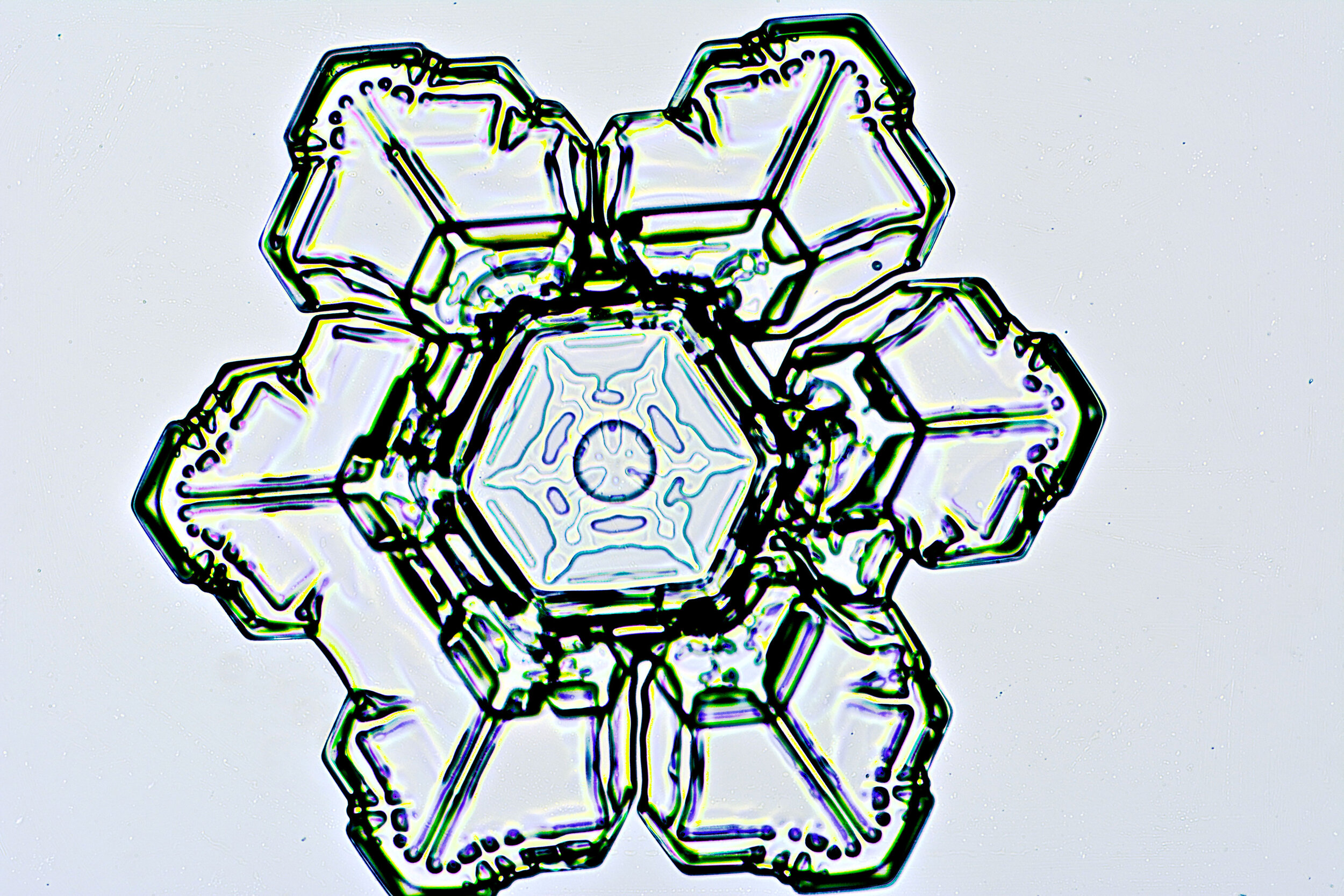


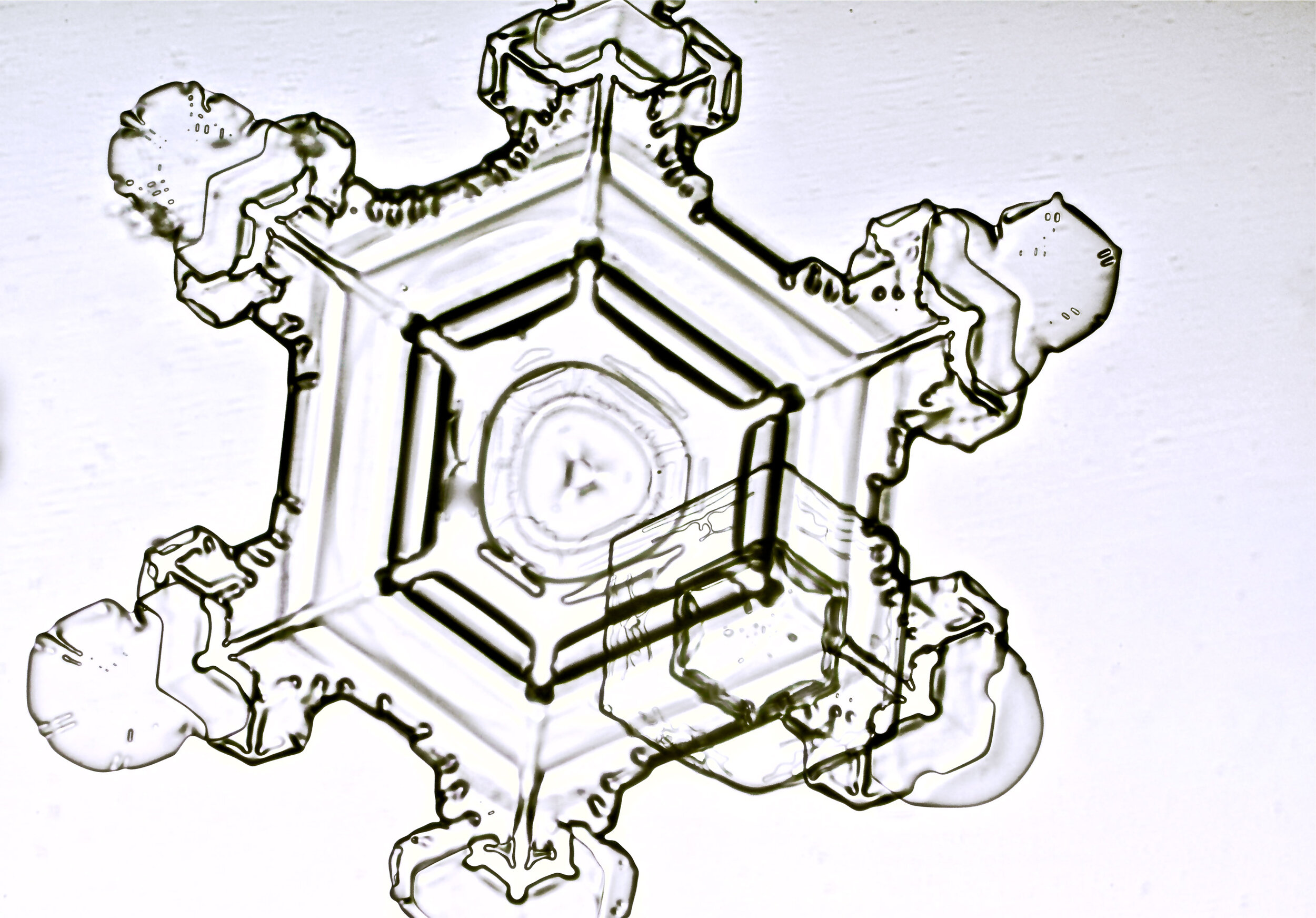
Process Notes:
I keep all of my equipment, except for my camera, outside on a covered porch so they are cold, to prevent the snow crystals from melting while I photograph them.

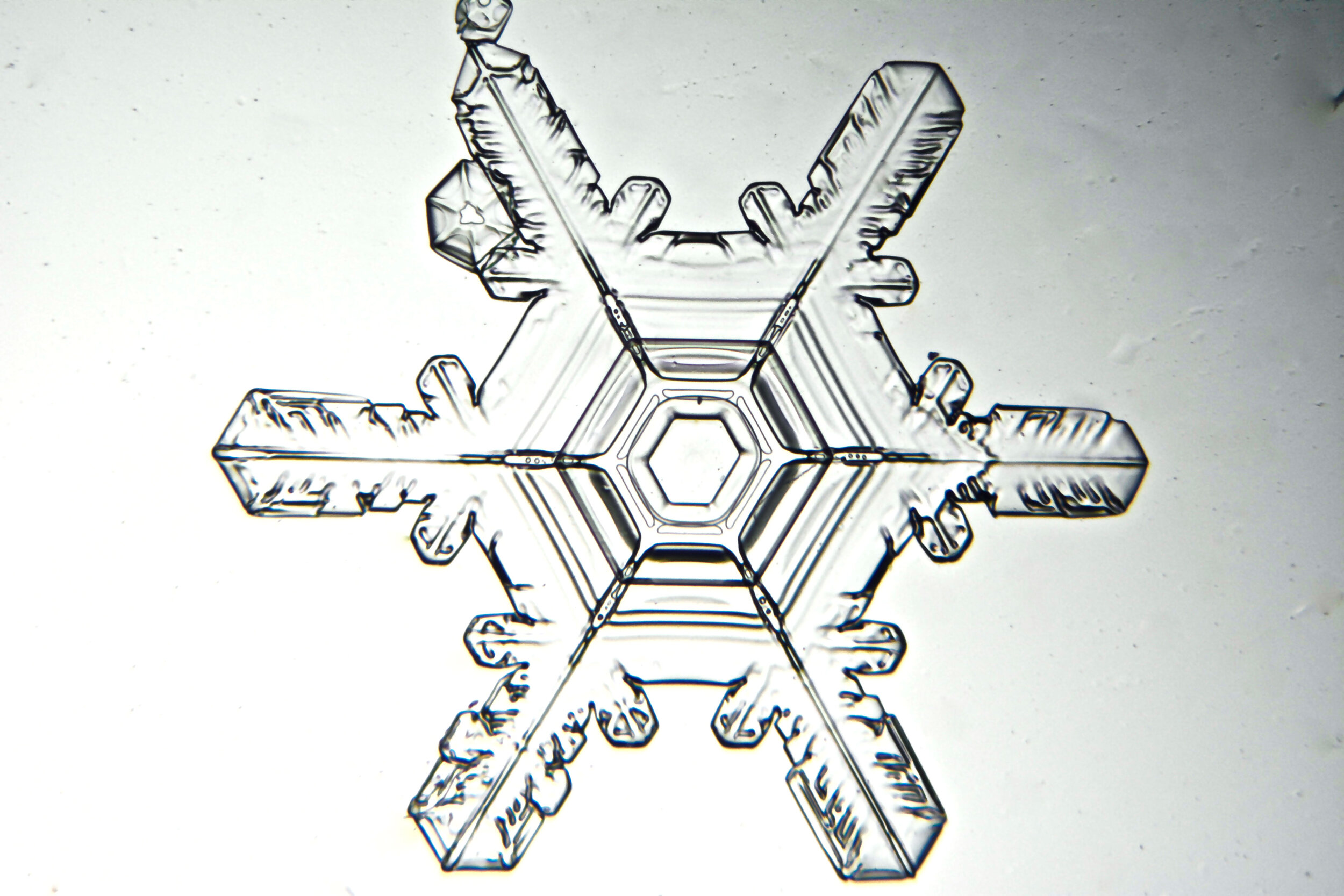
Stellar Dendrites
When we think of beautiful snow crystals, this is what we have in mind. In higher humidity conditions, when the branches grow longer, perhaps at least the diameter of the plate, the crystals are referred to as stellar dendrites. Stellar, in reference to stars, and dendrites, referring to branches (the scientific study of trees is called dendrology). These branches can become long, and rather ornate, and while not always perfect replicas, the six branches of an intact stellar dendrite (that is, one not damaged by collisions) exhibit marvelous symmetry because they are sharing the ride through identical conditions as they grow.
Plates 5.1 - 5.11
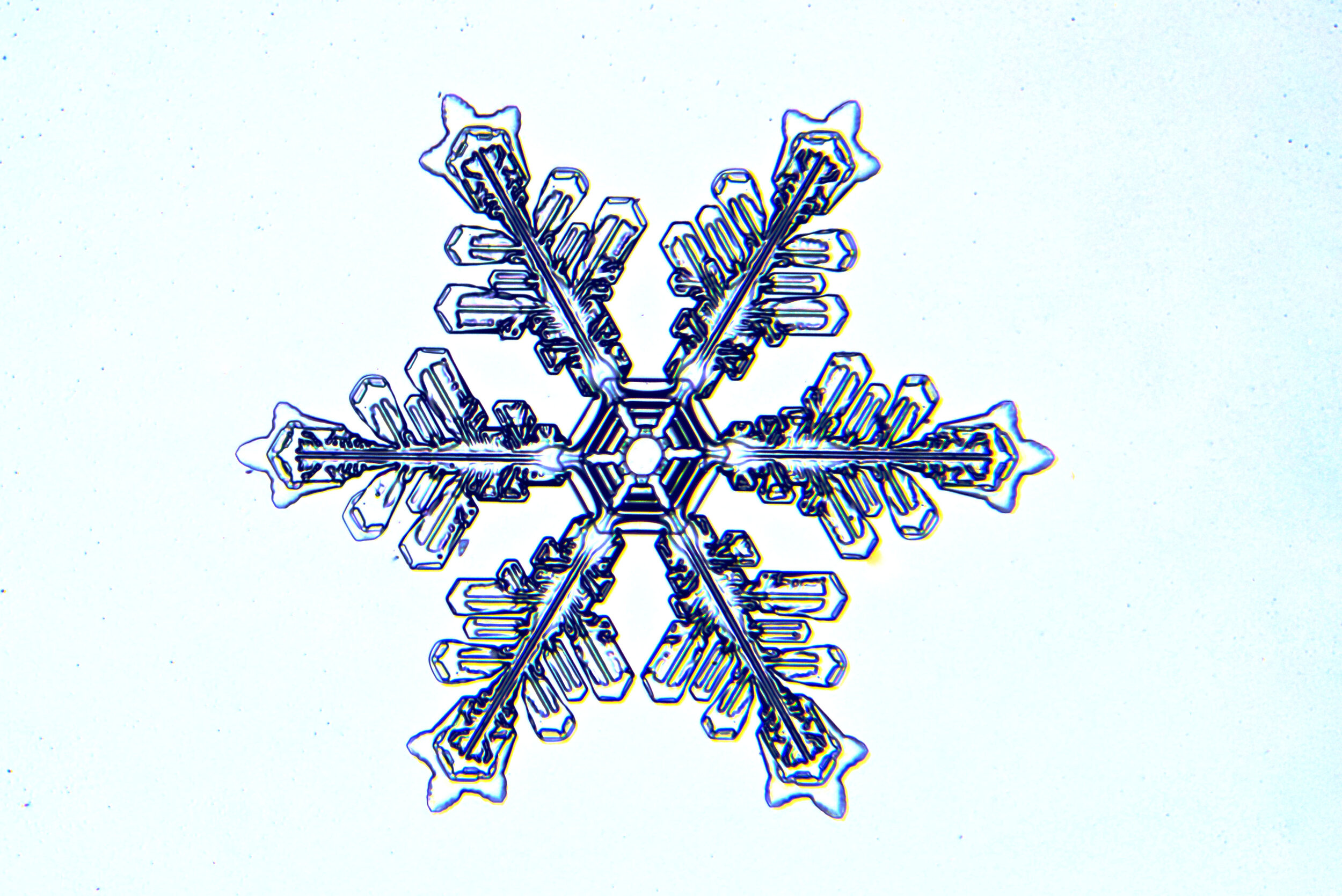










Process Notes:
I catch falling snow on 3”x4” glass plates that are sitting on a board covered with black velvet, which holds 8 plates. By naked eye, I can tell which plates contain promising crystals, and I move those plates onto the microscope for magnified examination and photography.
Plates 5.12 - 5.23









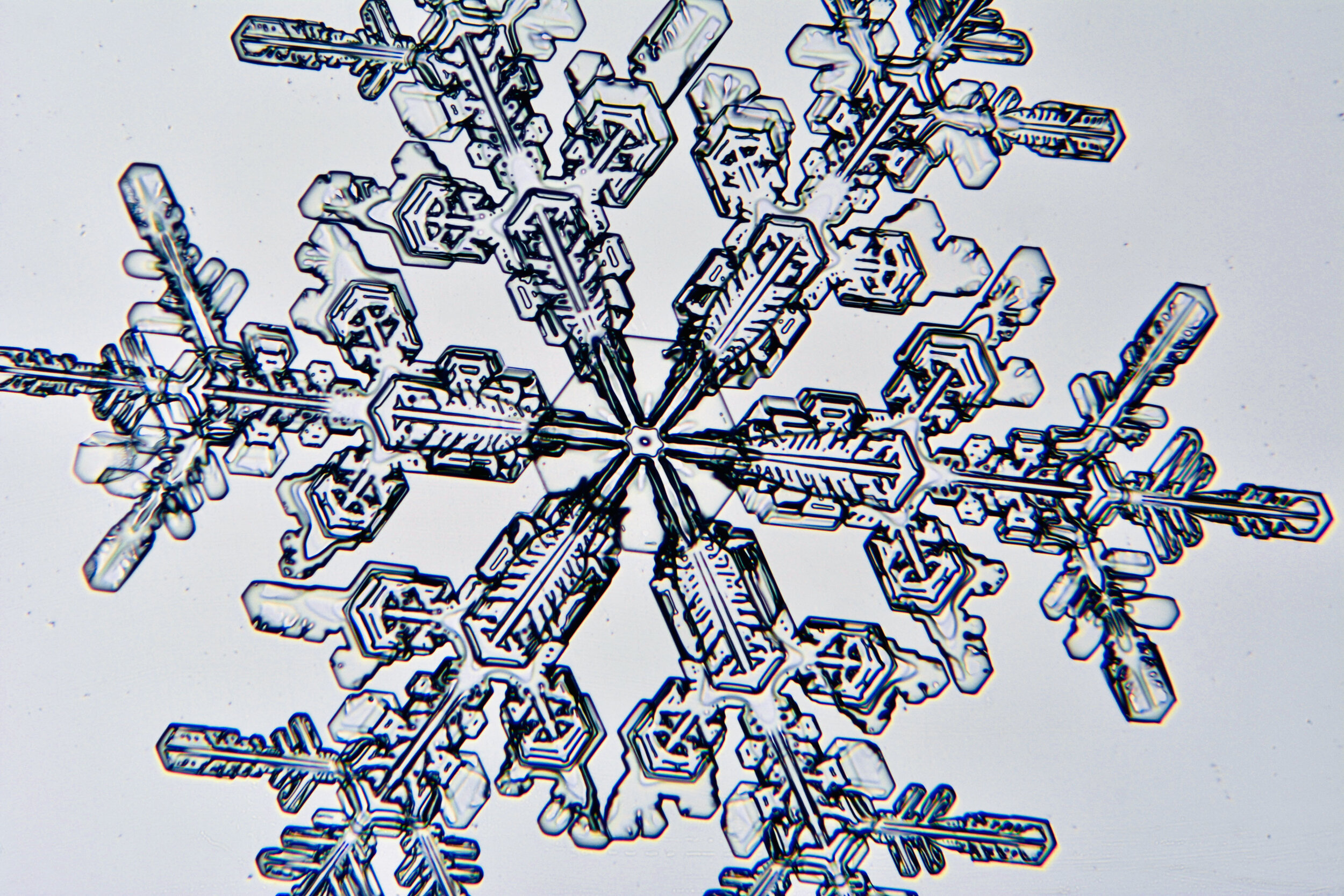
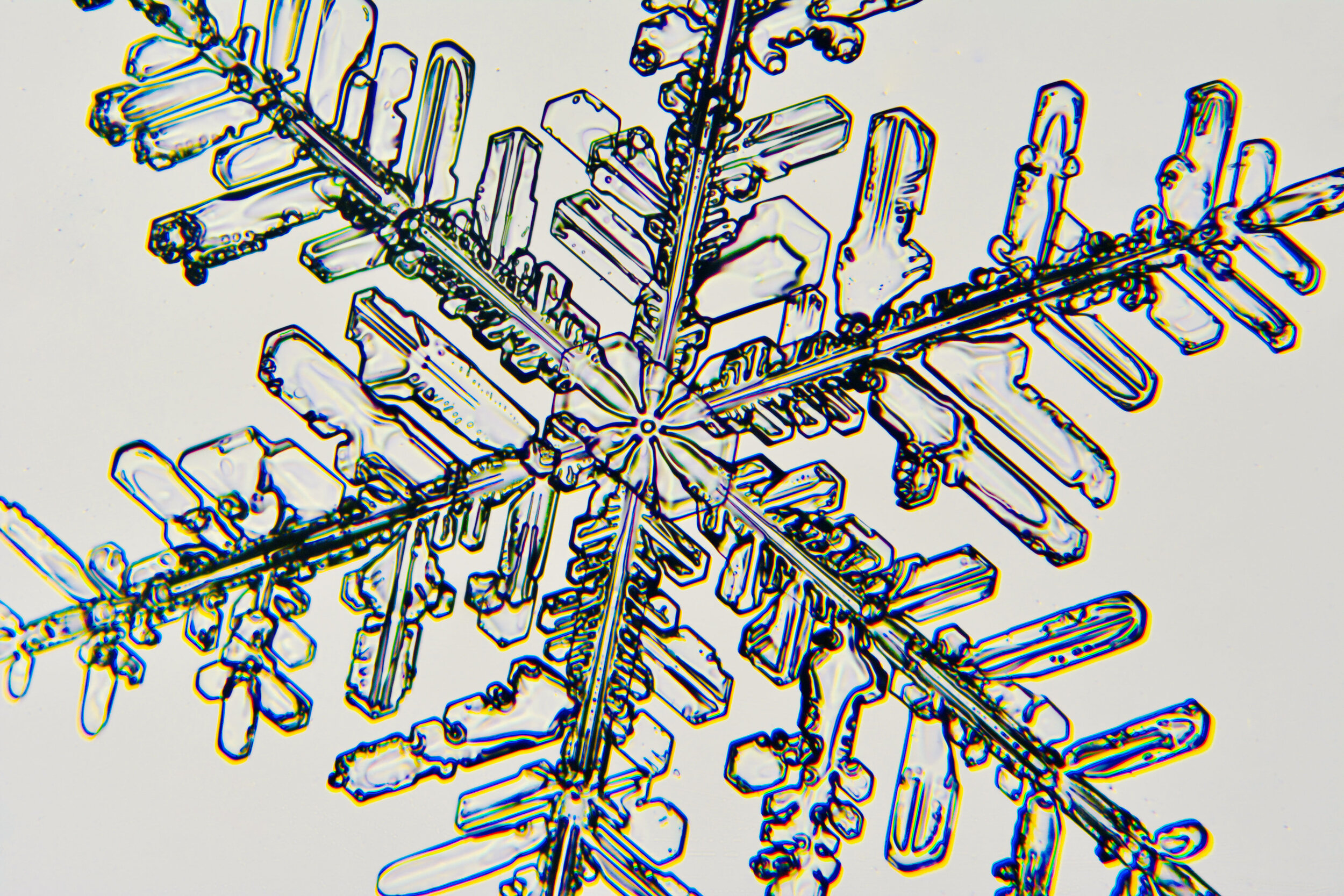

Process notes:
I have found that the temperature has to be ≤ 26°F to prevent the snow crystals from melting on the glass plates. Above that temperature, individual crystals quickly turn into tiny dots of water.
Plates 5.24 - 5.34
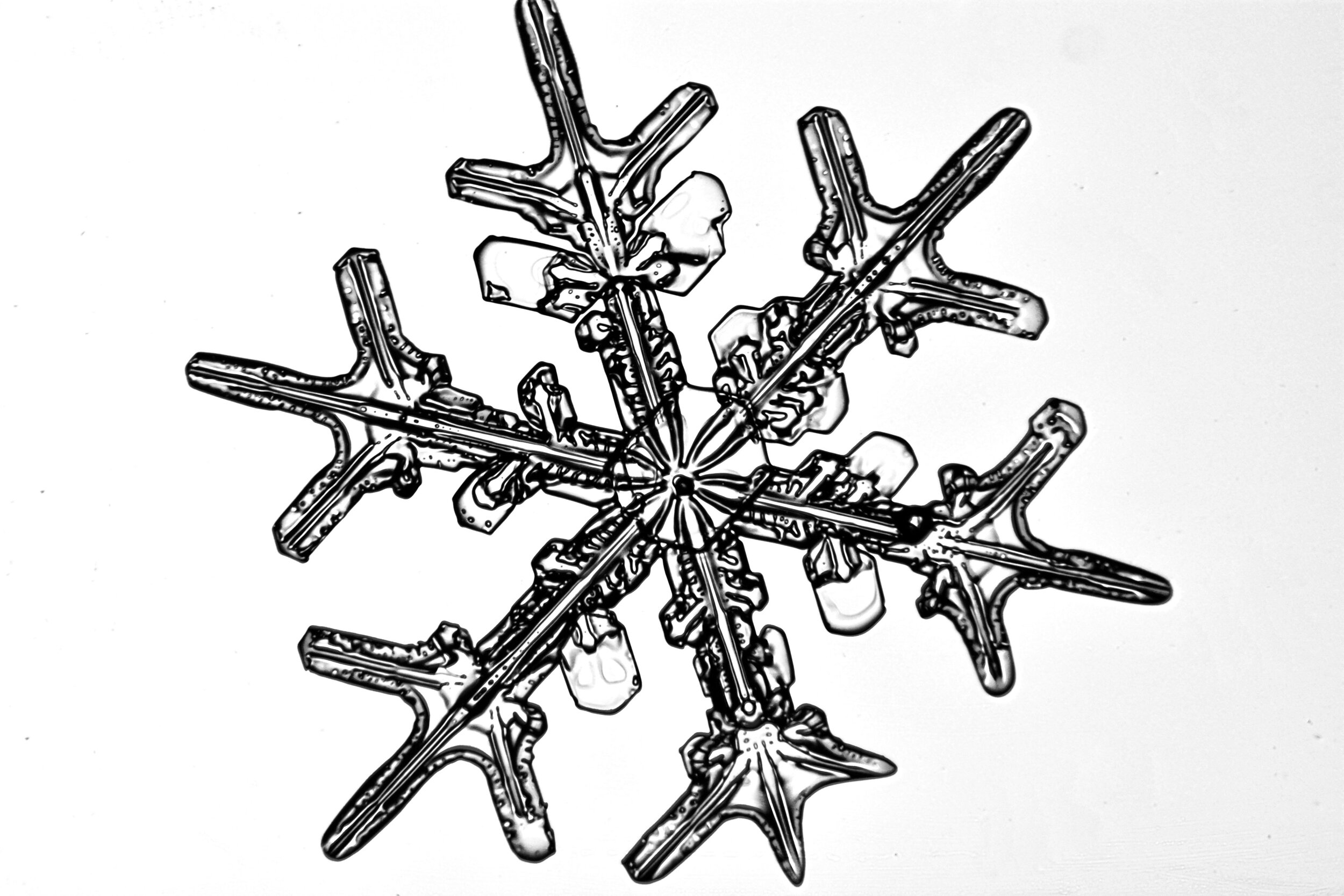
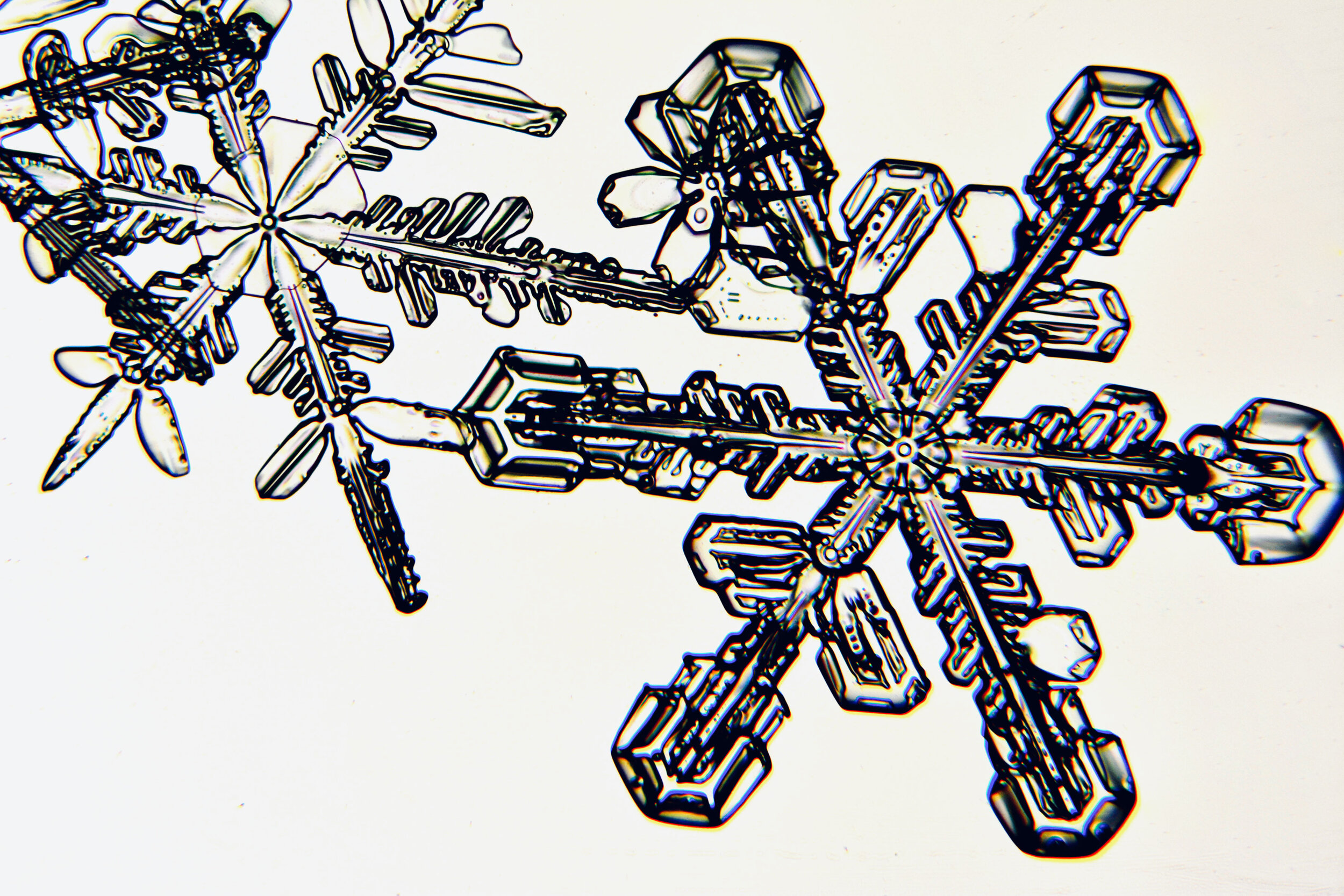
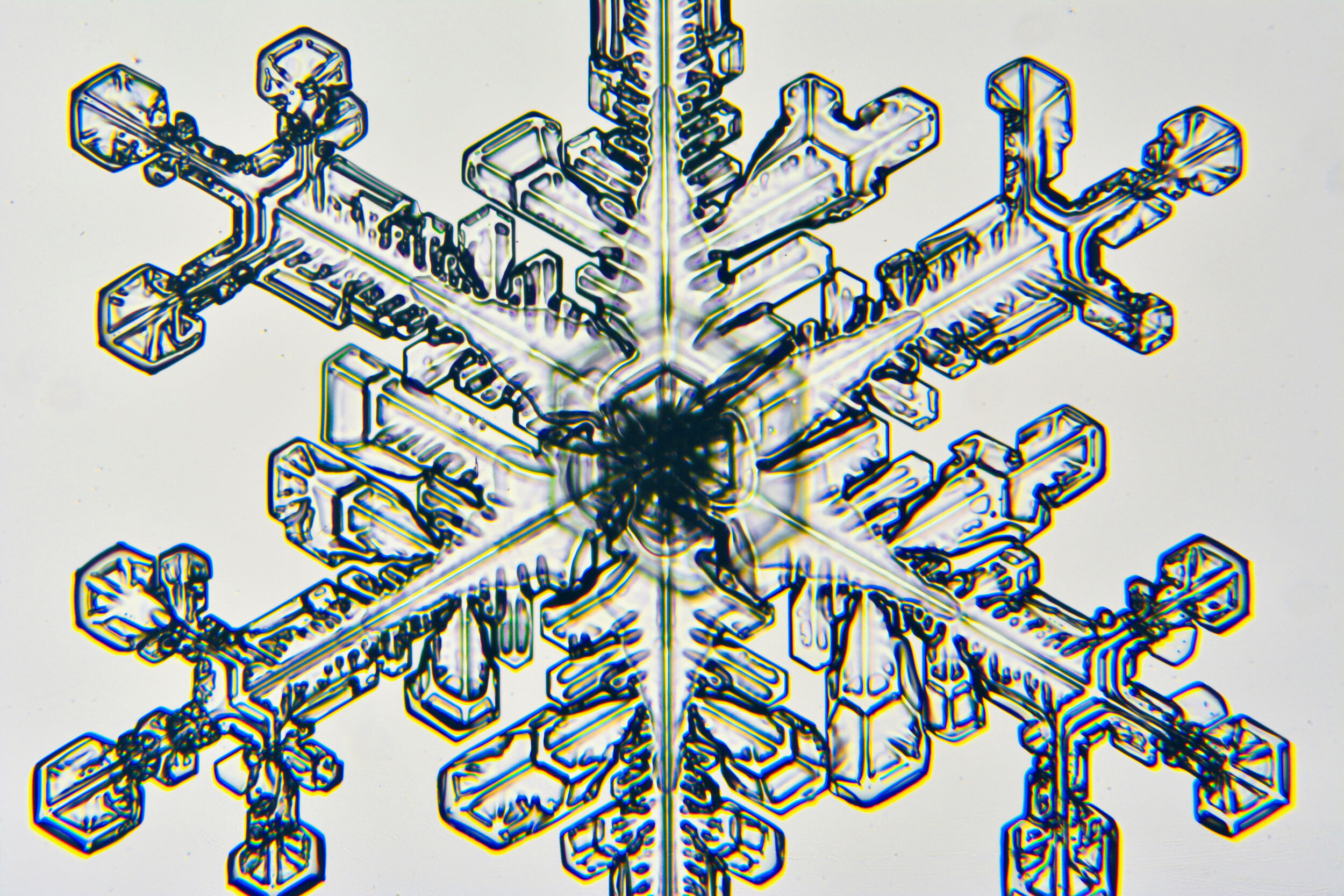
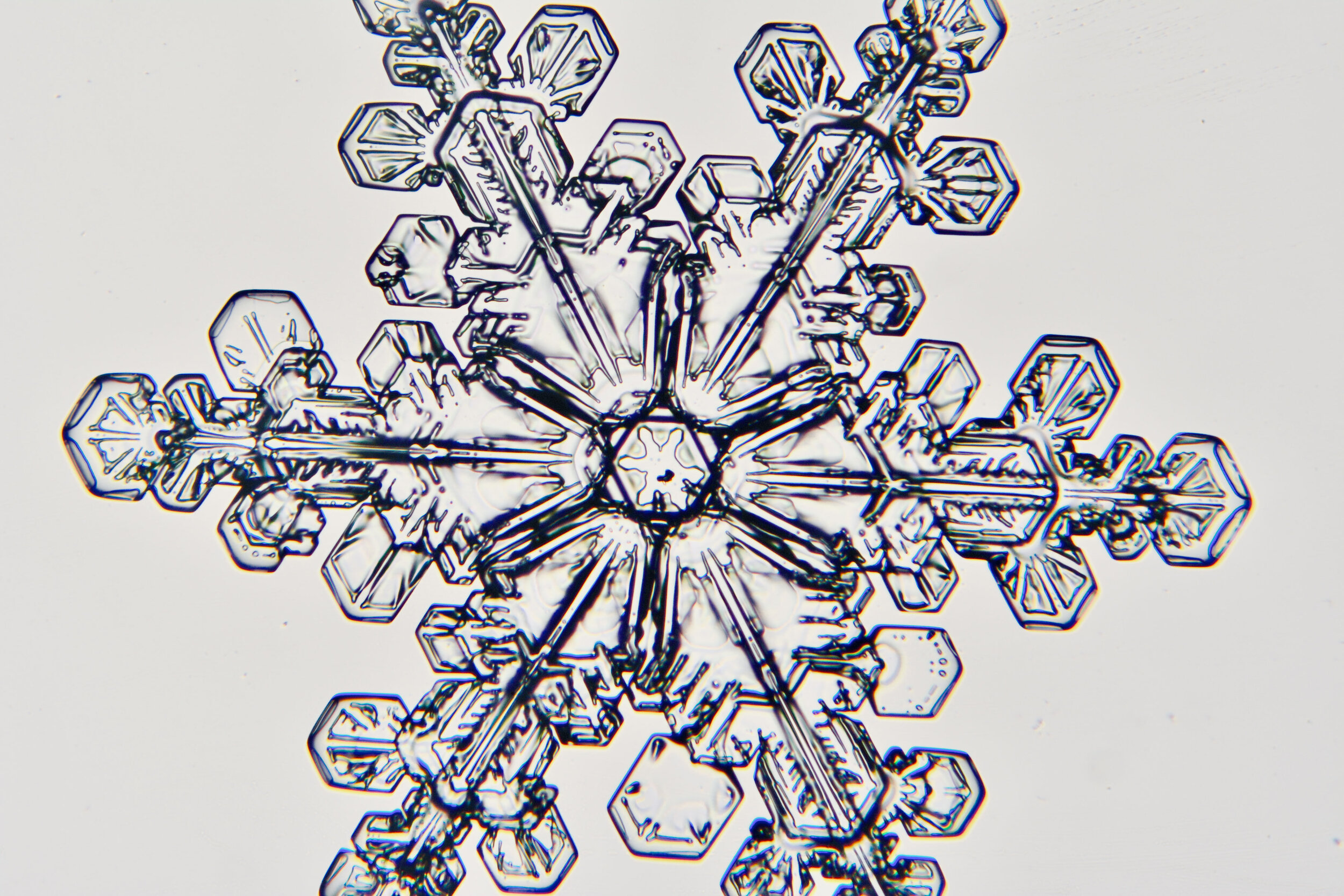
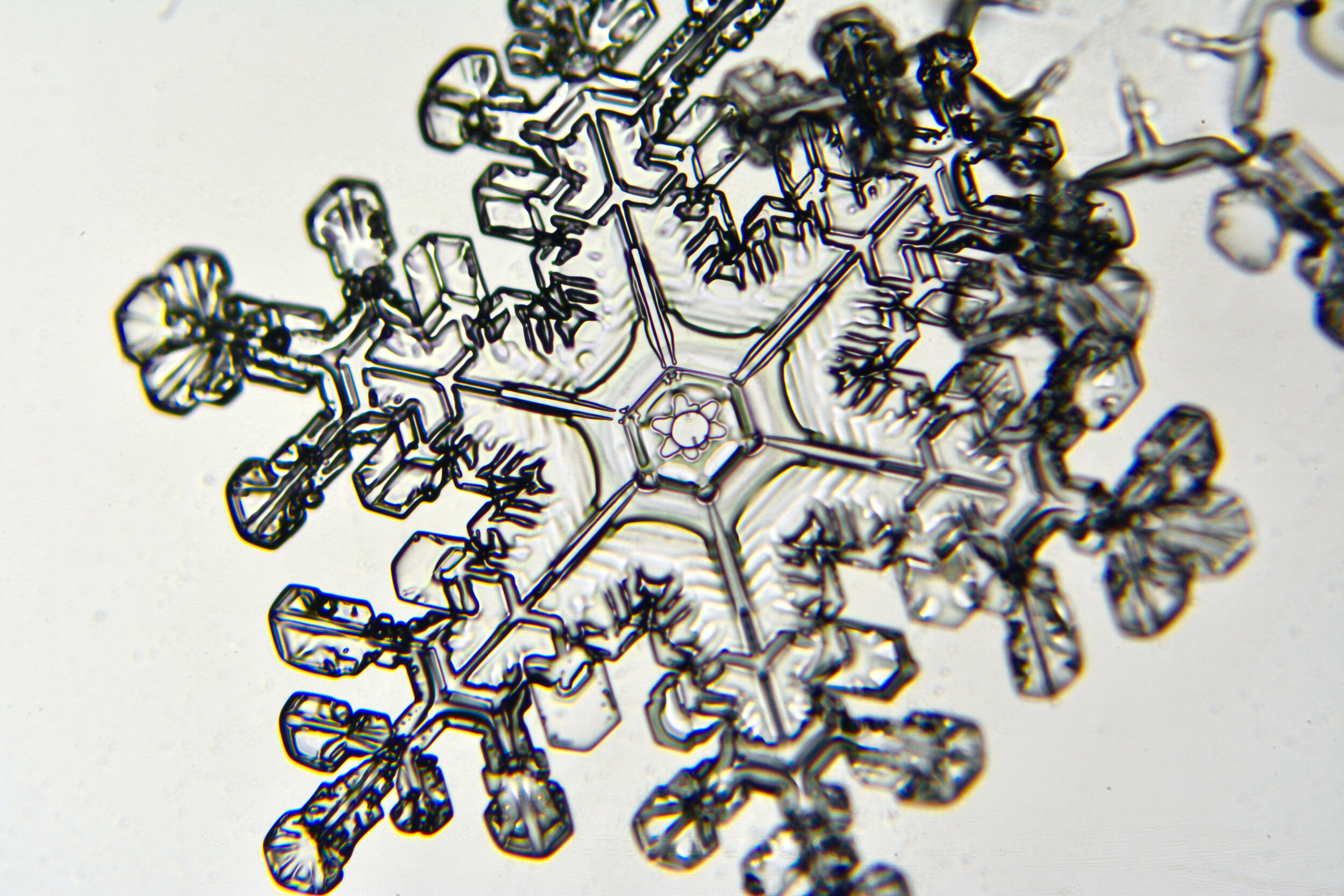
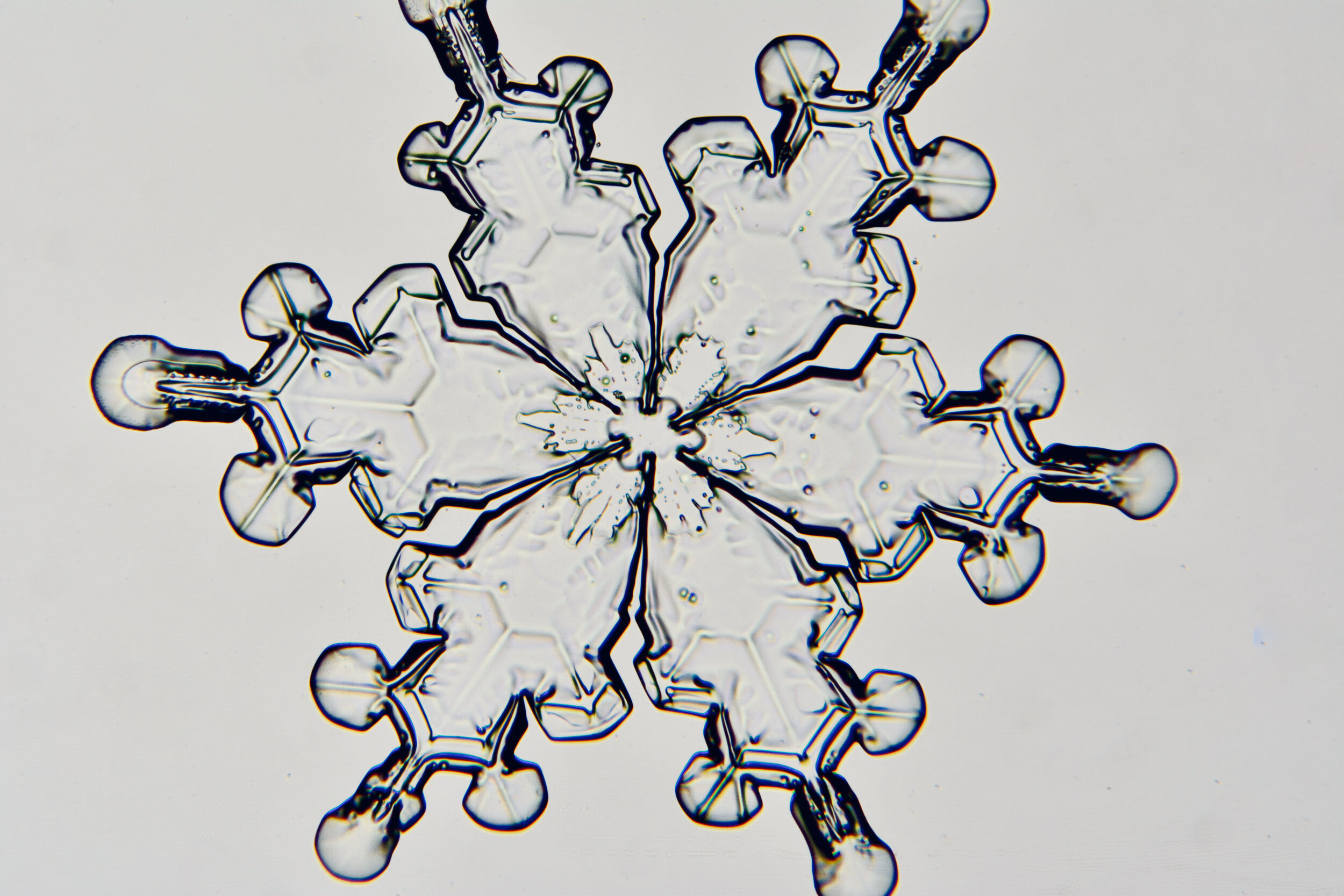
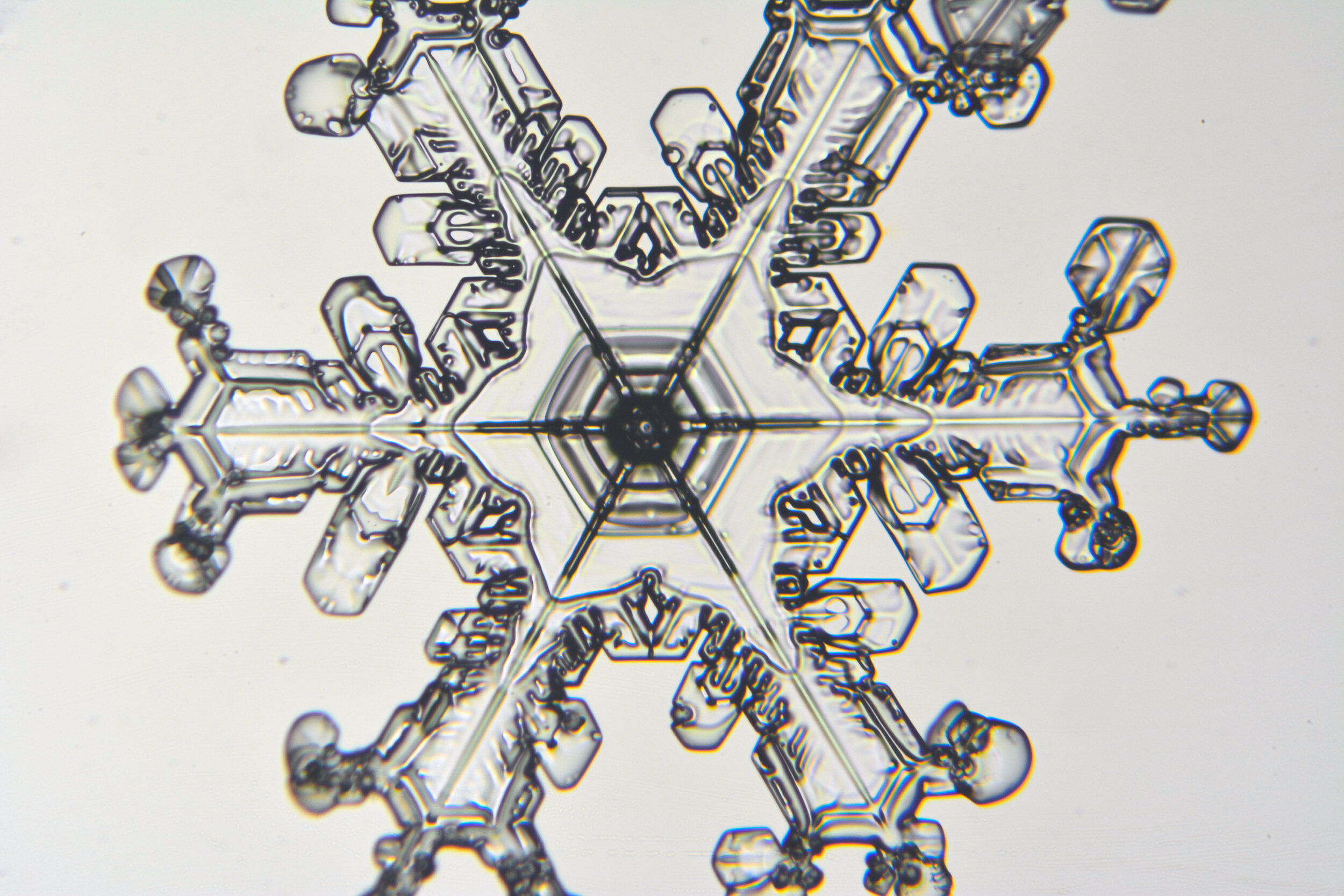

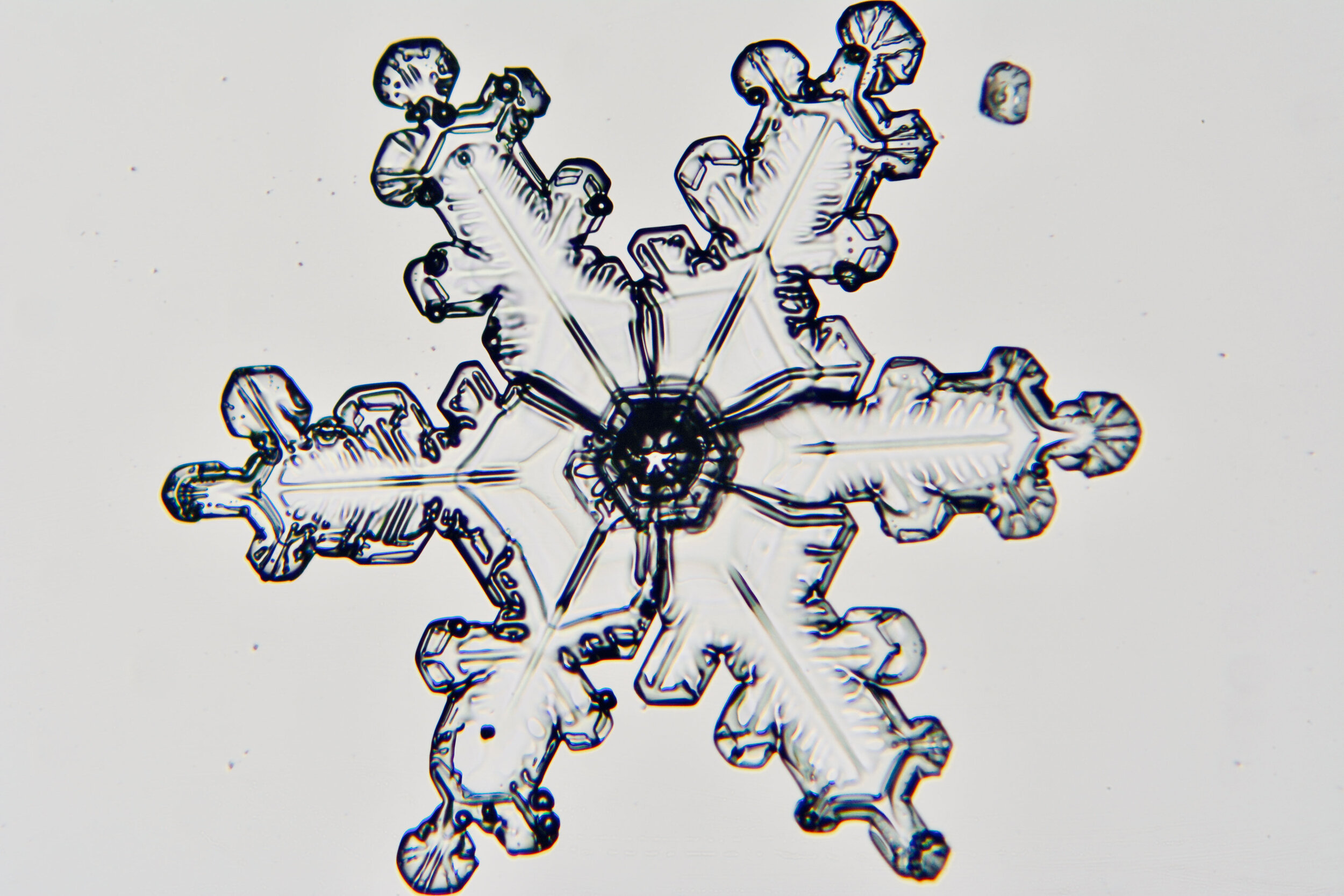
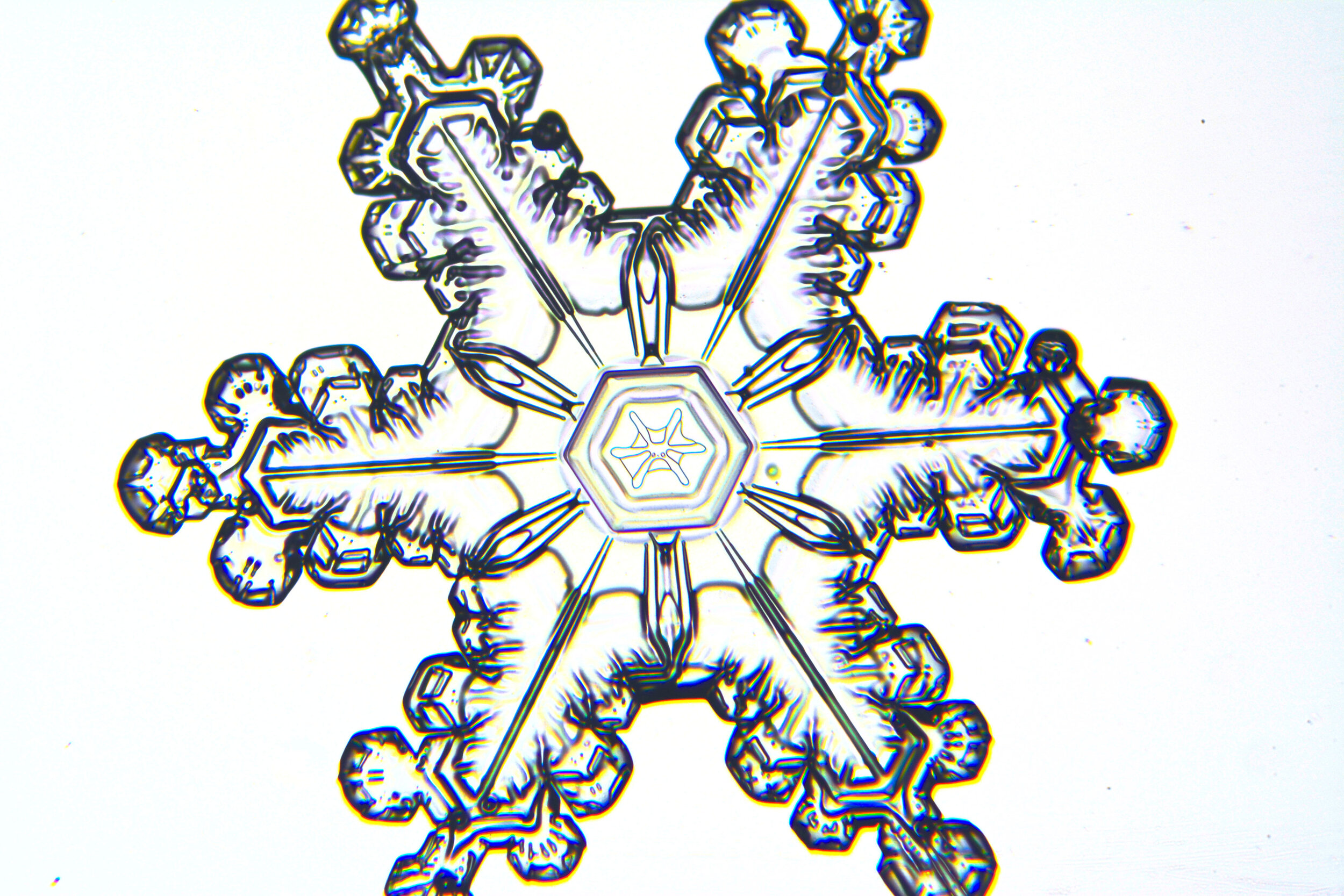

Process Notes:
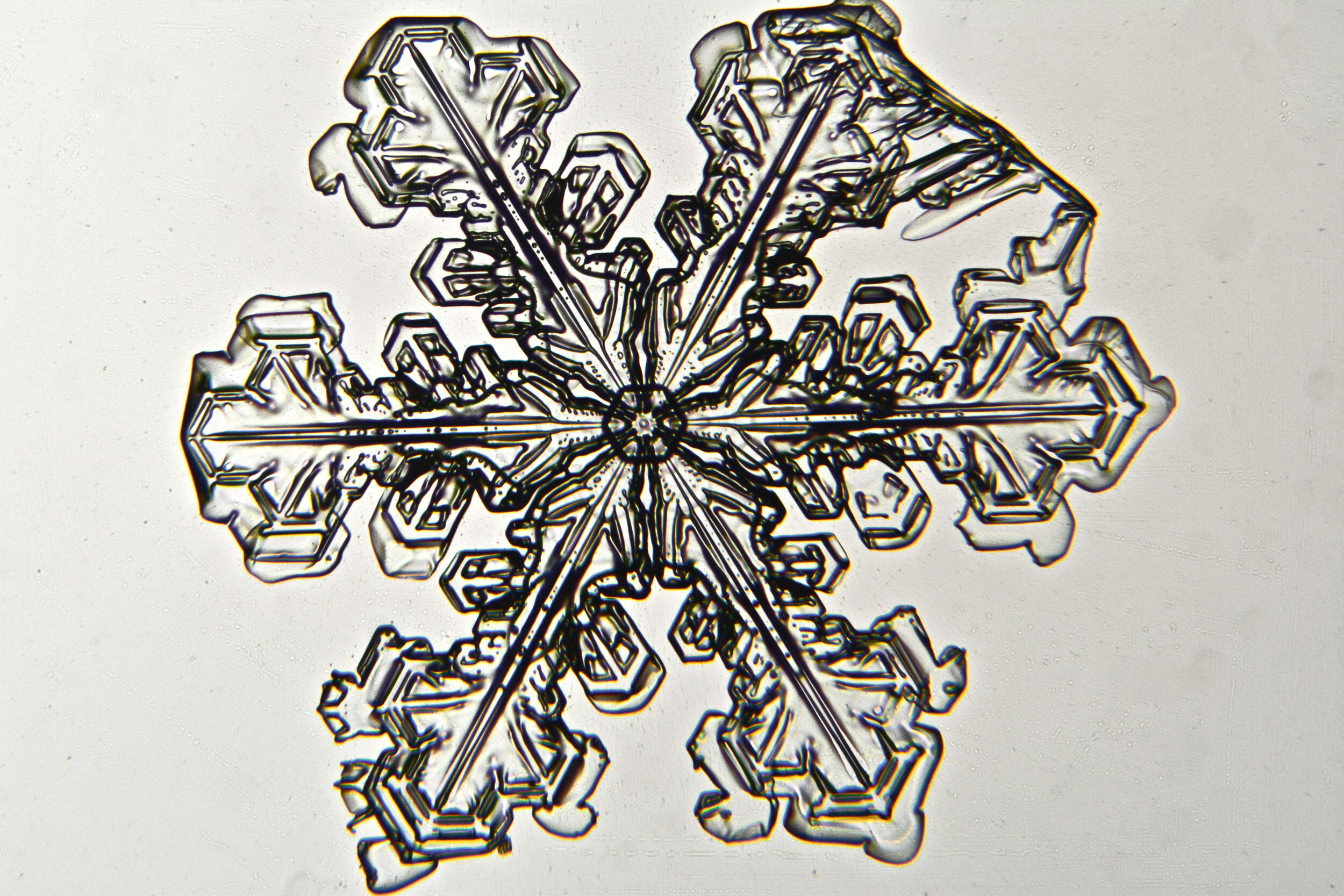
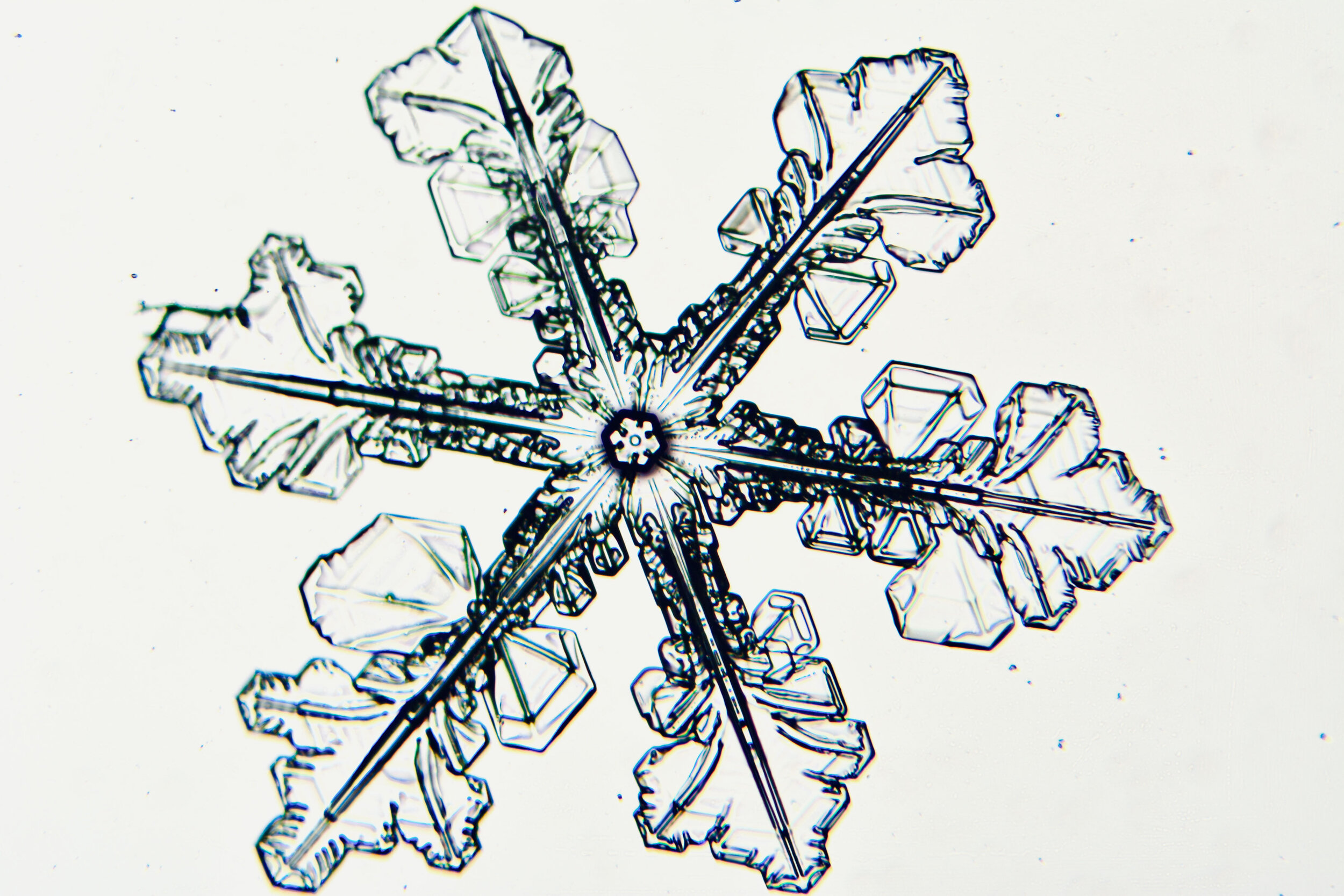
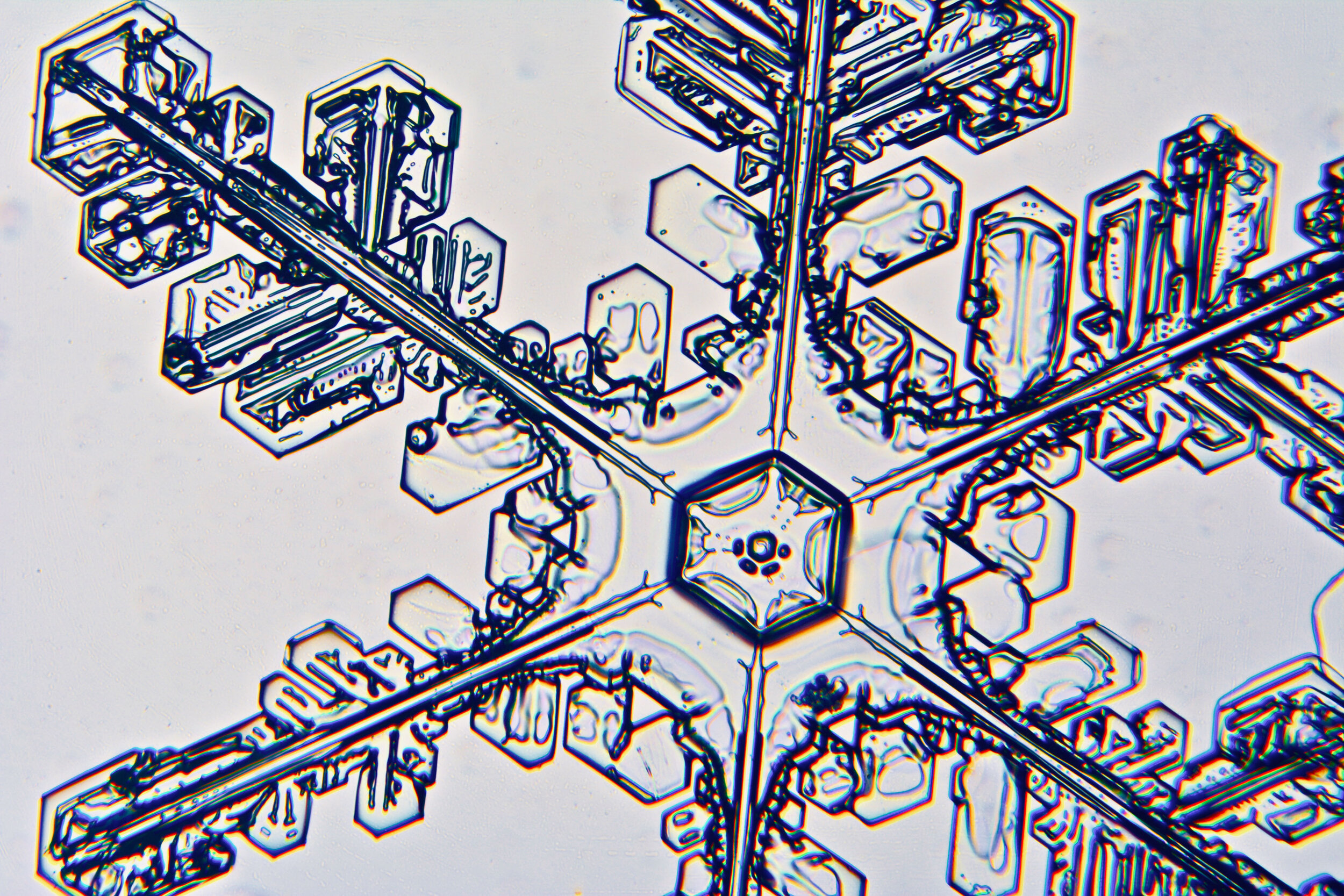
Even at very cold temperatures, the delicate tips of the crystals shrink away, sometimes within seconds, due to sublimation (water transforming directly from a solid to a gaseous state). This effect can be observed as rounded edges on the tips of the dendrites on some of the crystals shown here.
I wish I could talk to “Snowflake” Bentley about sublimation because his photographs were taken with exposures of 8-100 seconds, yet there is little or no sign of dissipation.
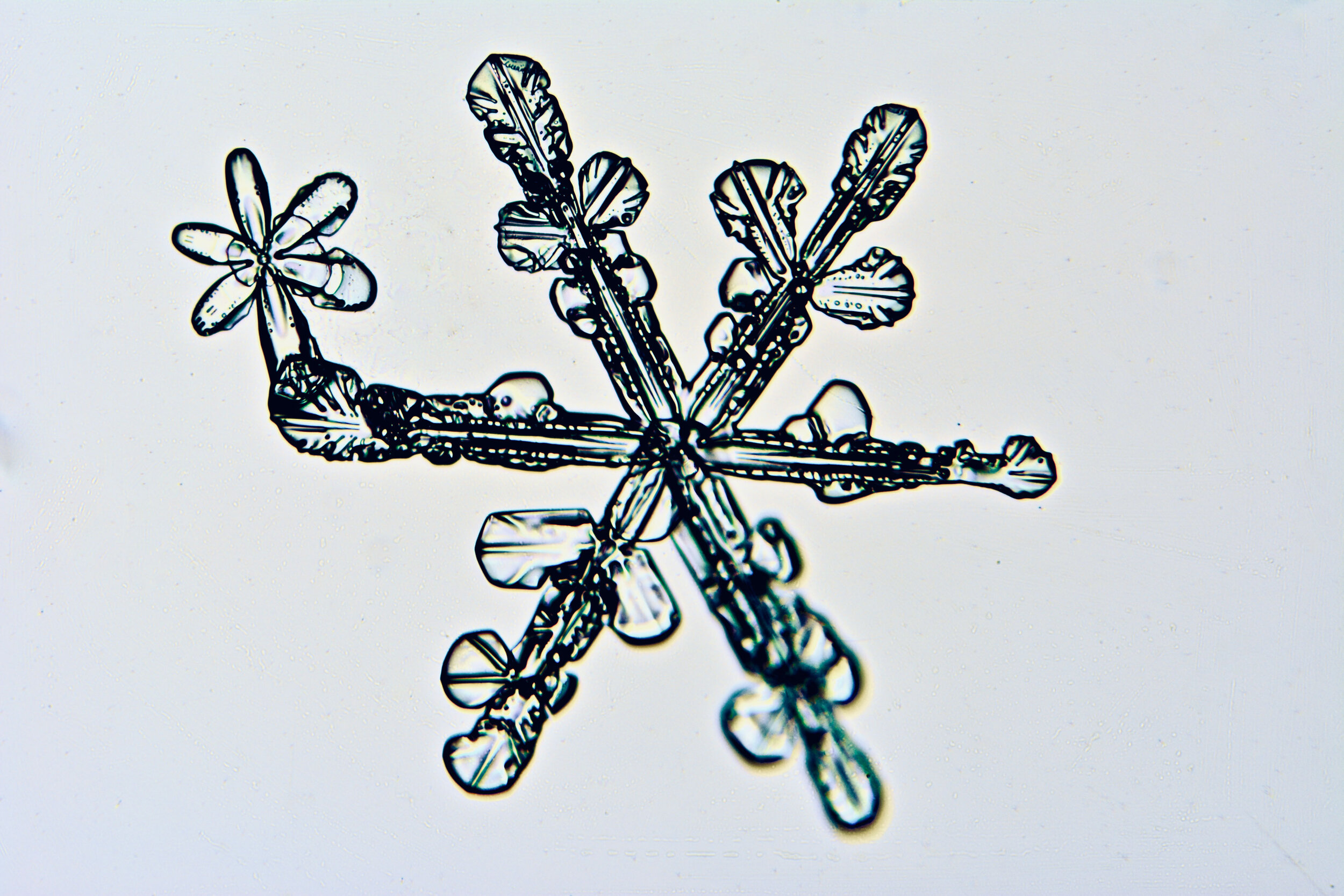
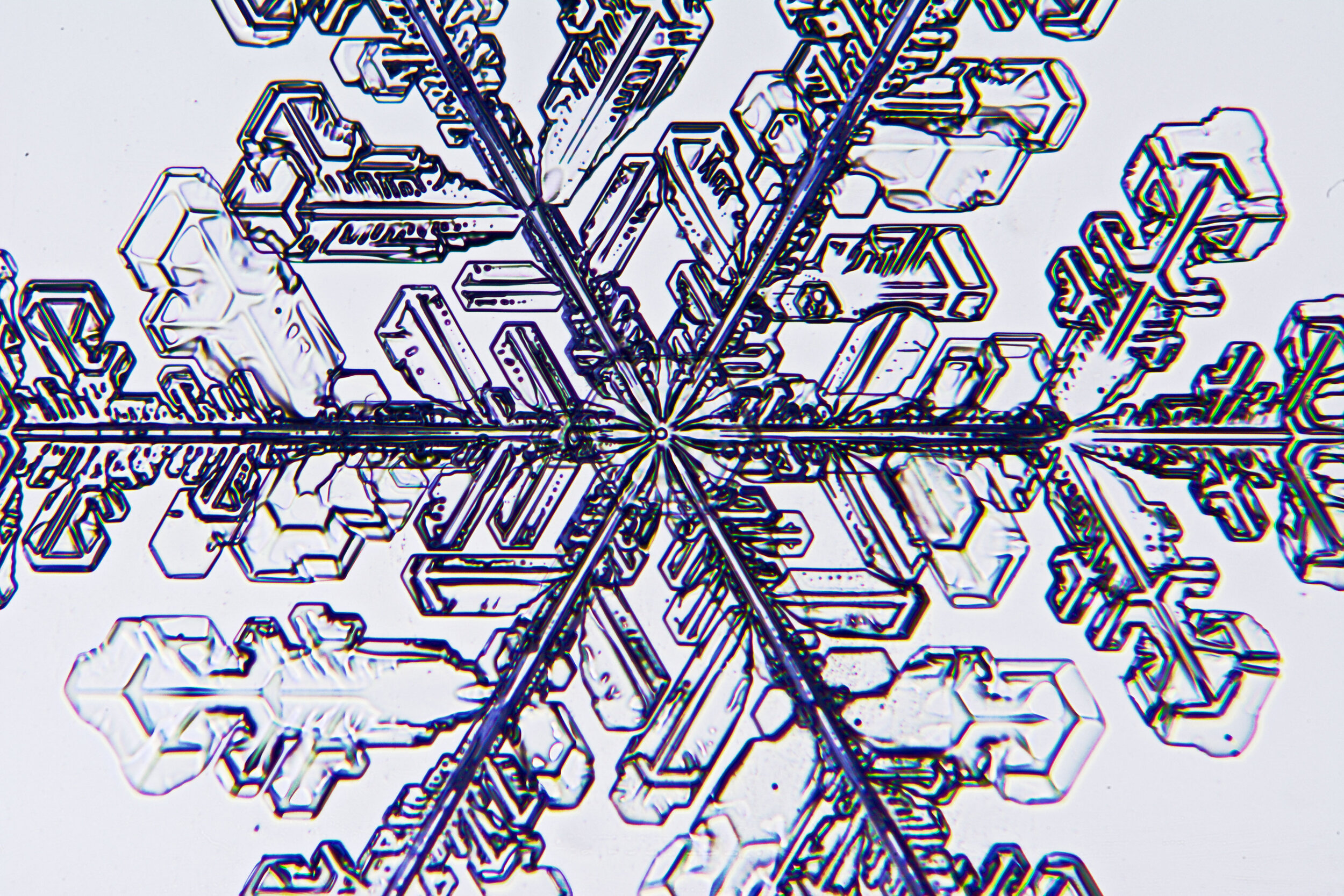
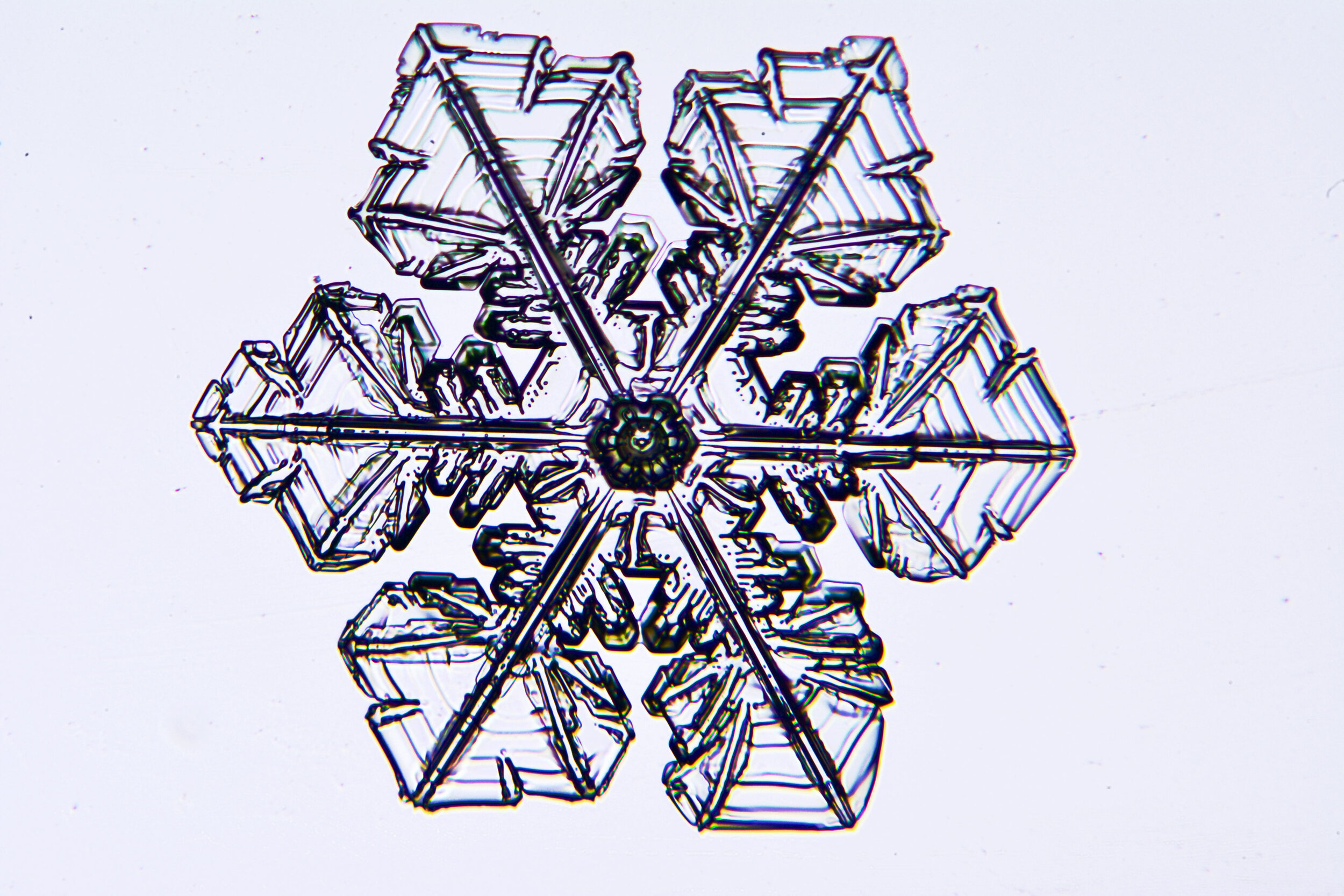
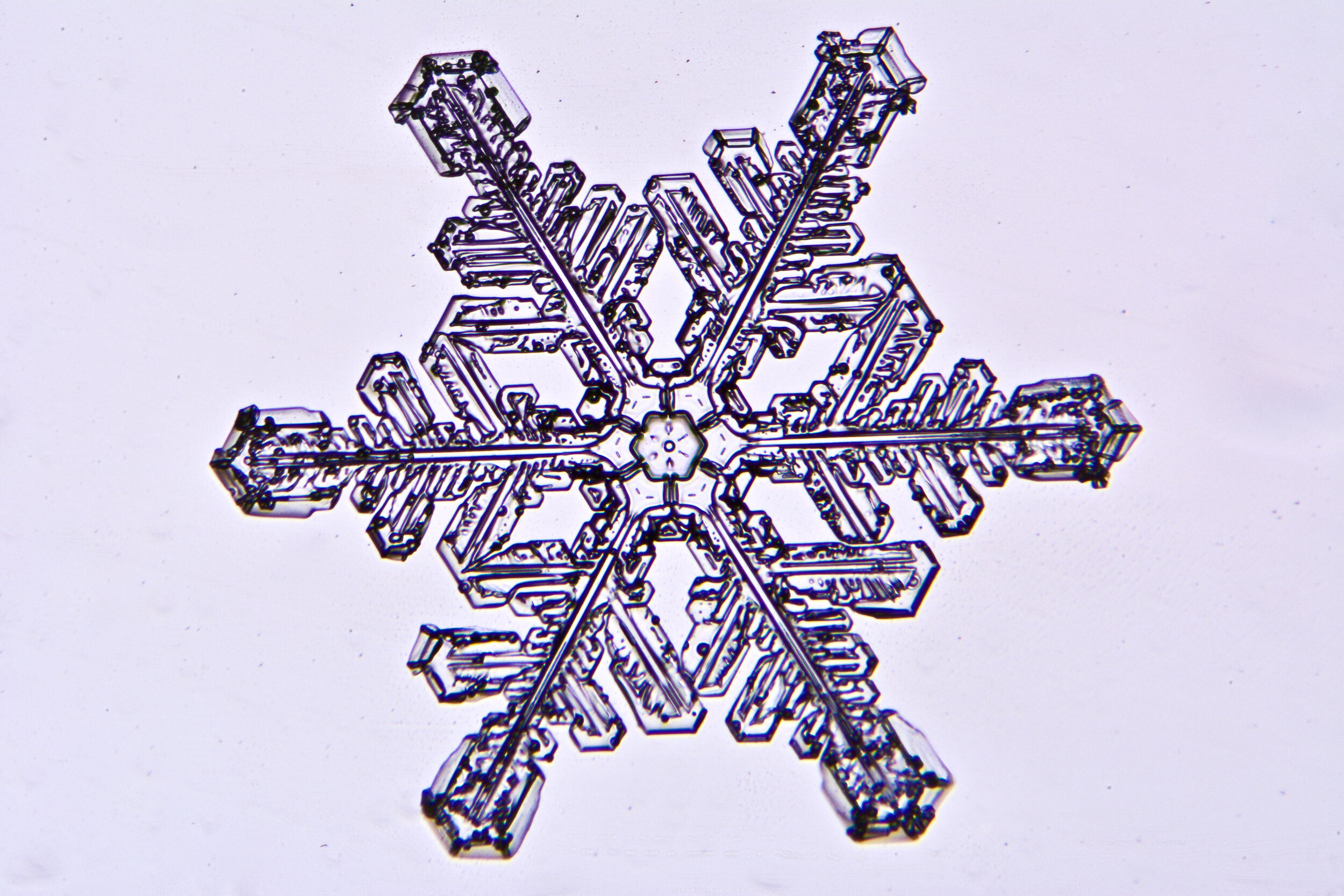
Plates 5.35 - 5.46

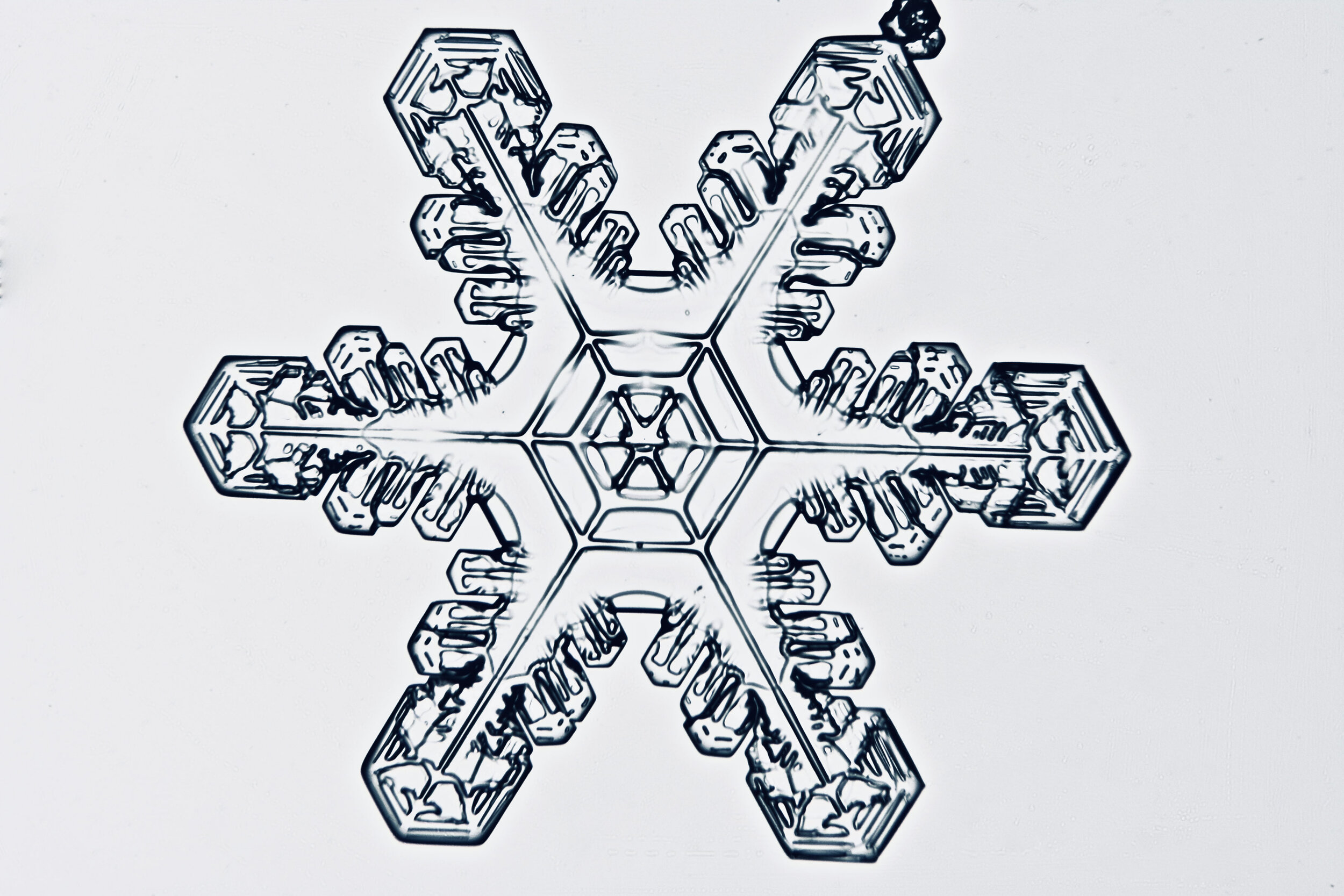
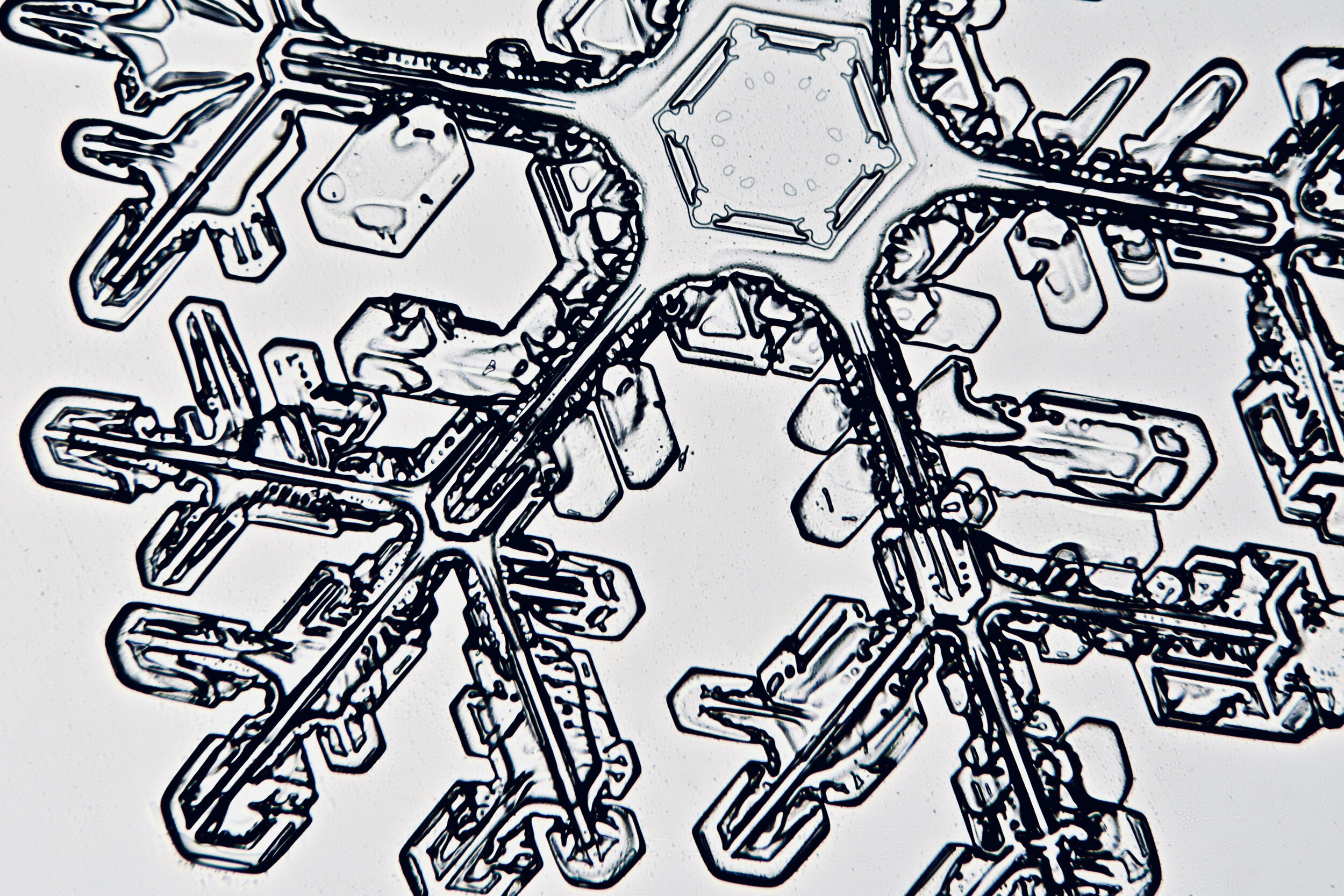
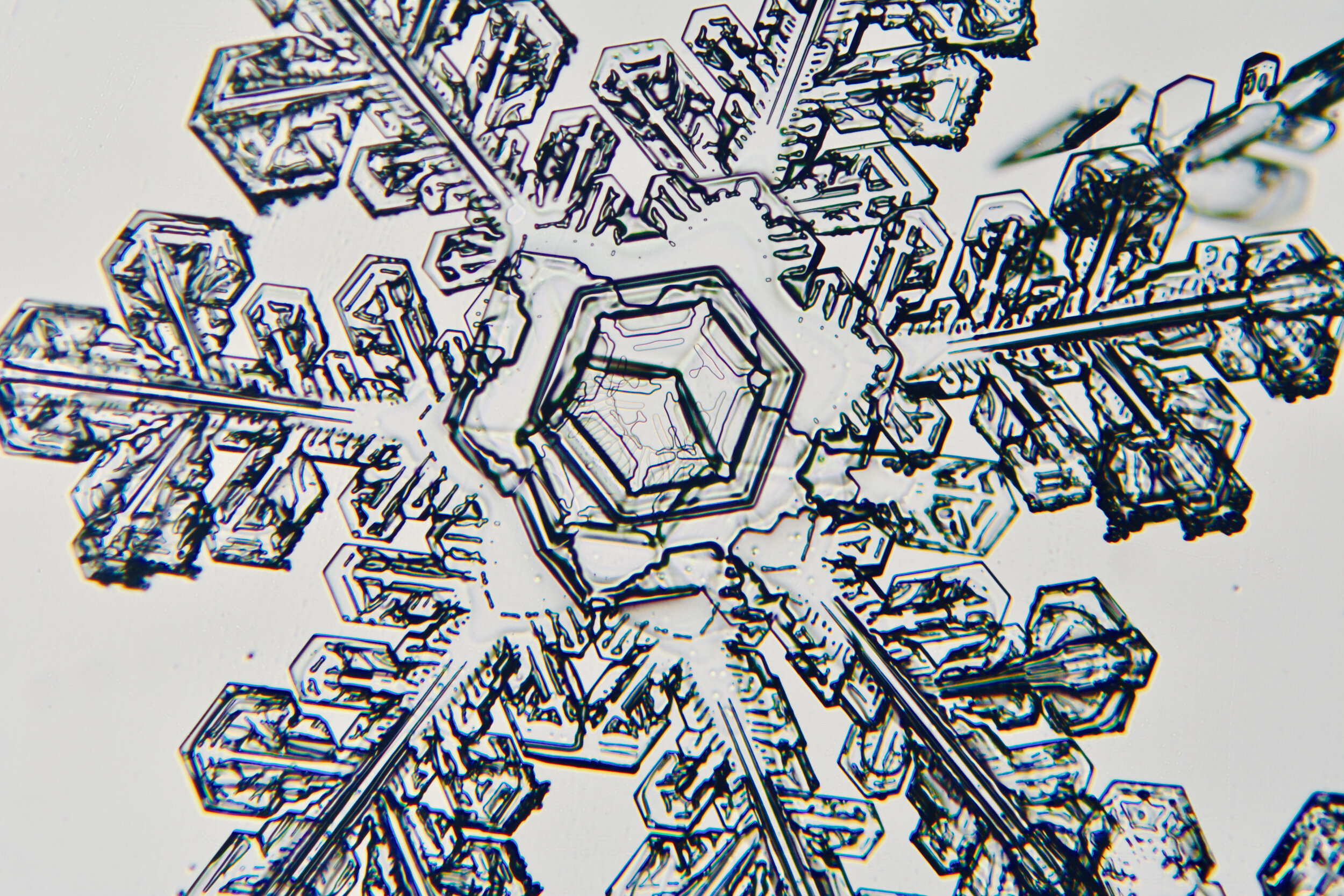




Process Notes:
During a snow storm, when conditions are right for forming crystals (rather than just gravel), most snow crystals are damaged by the time they reach the ground from being buffeted by the wind and from crashing into each other.
Plates 5.47 - 5.57
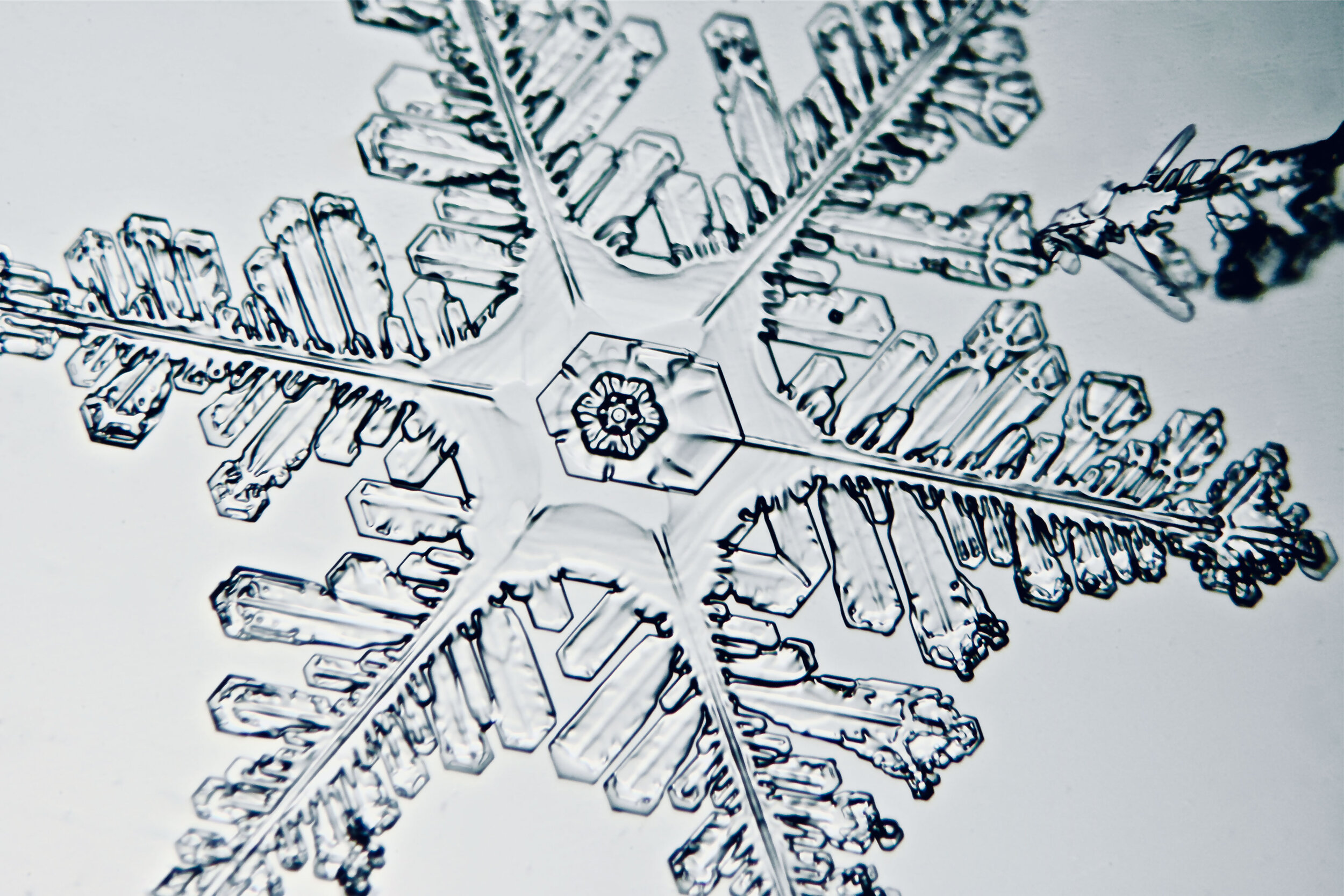
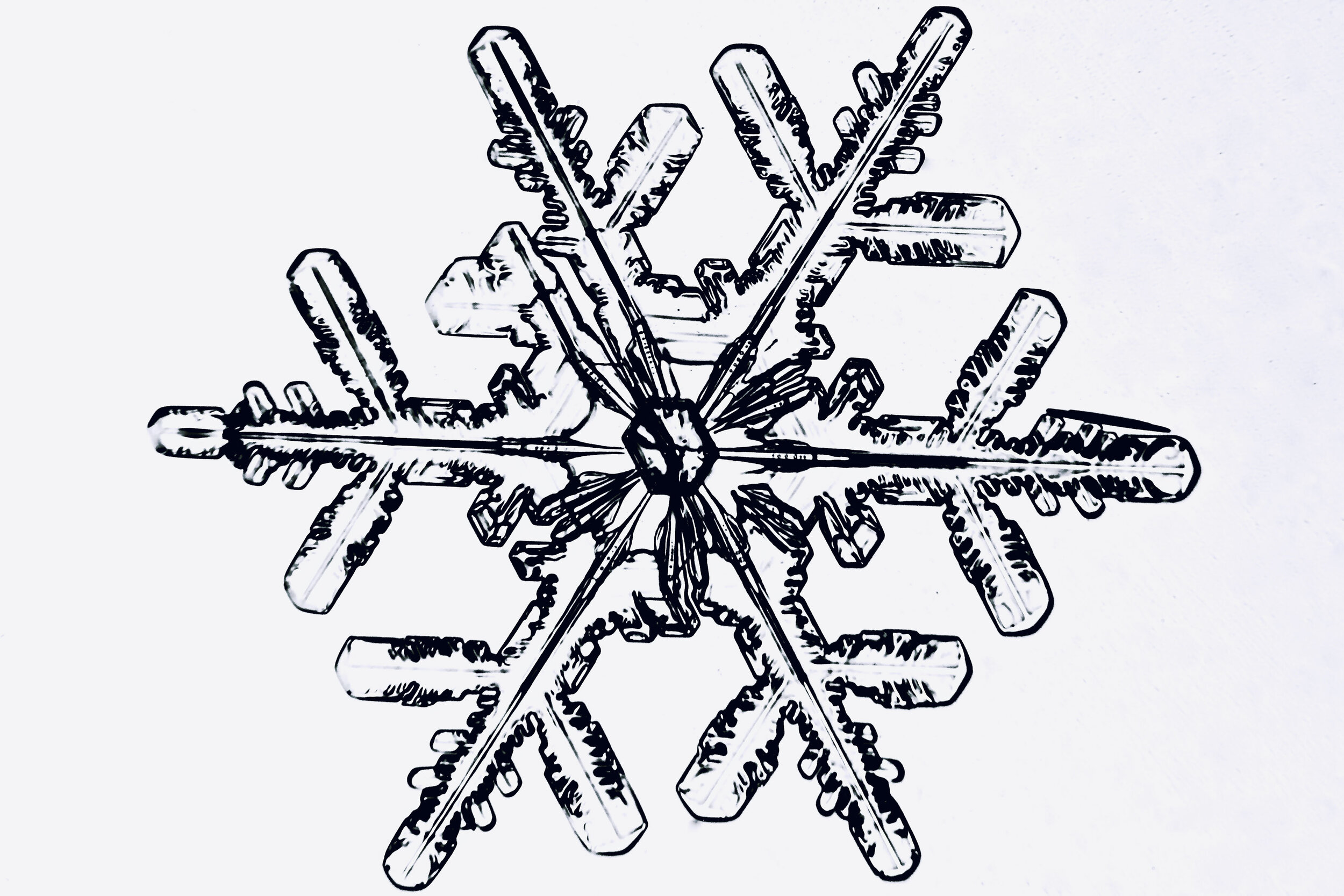

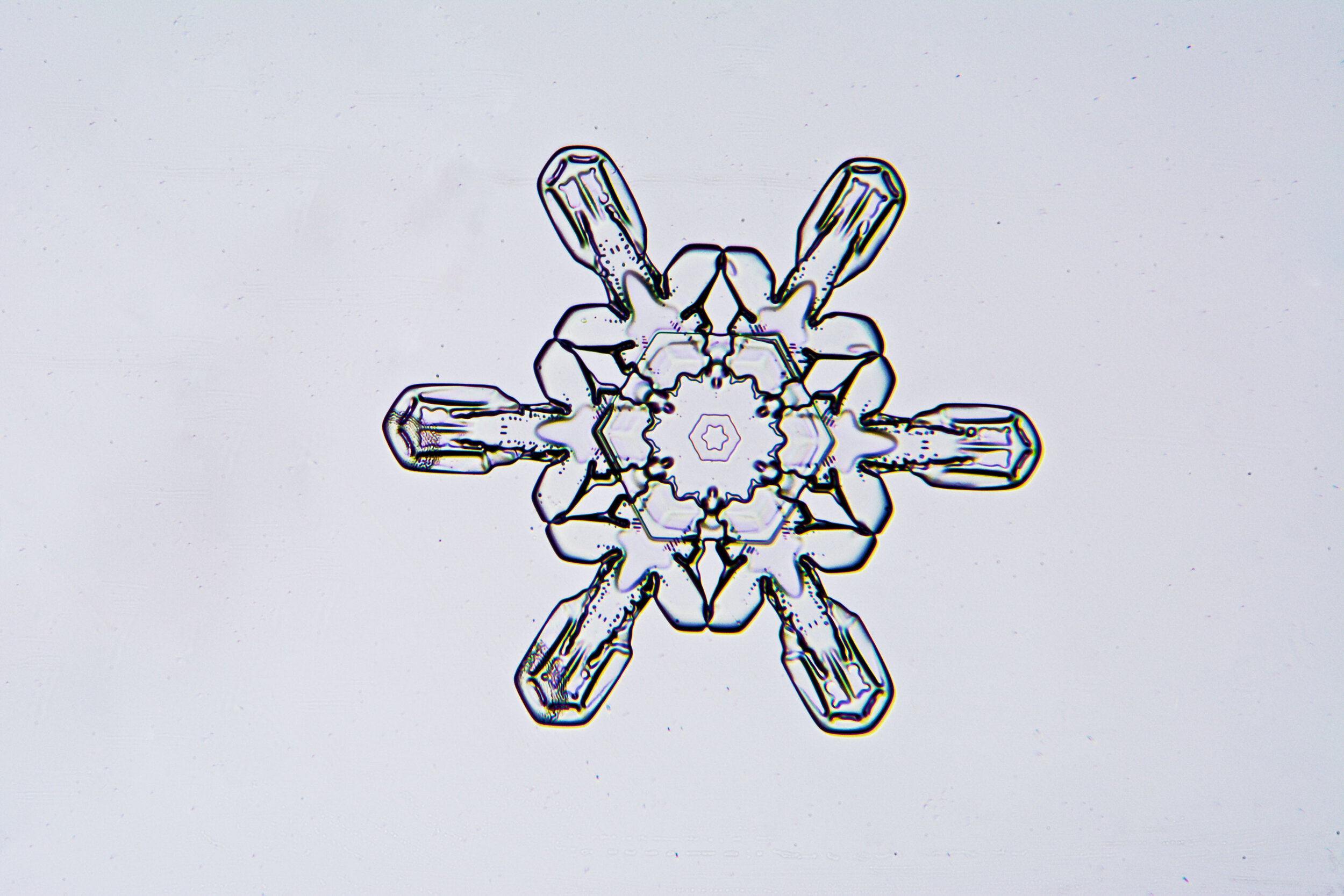
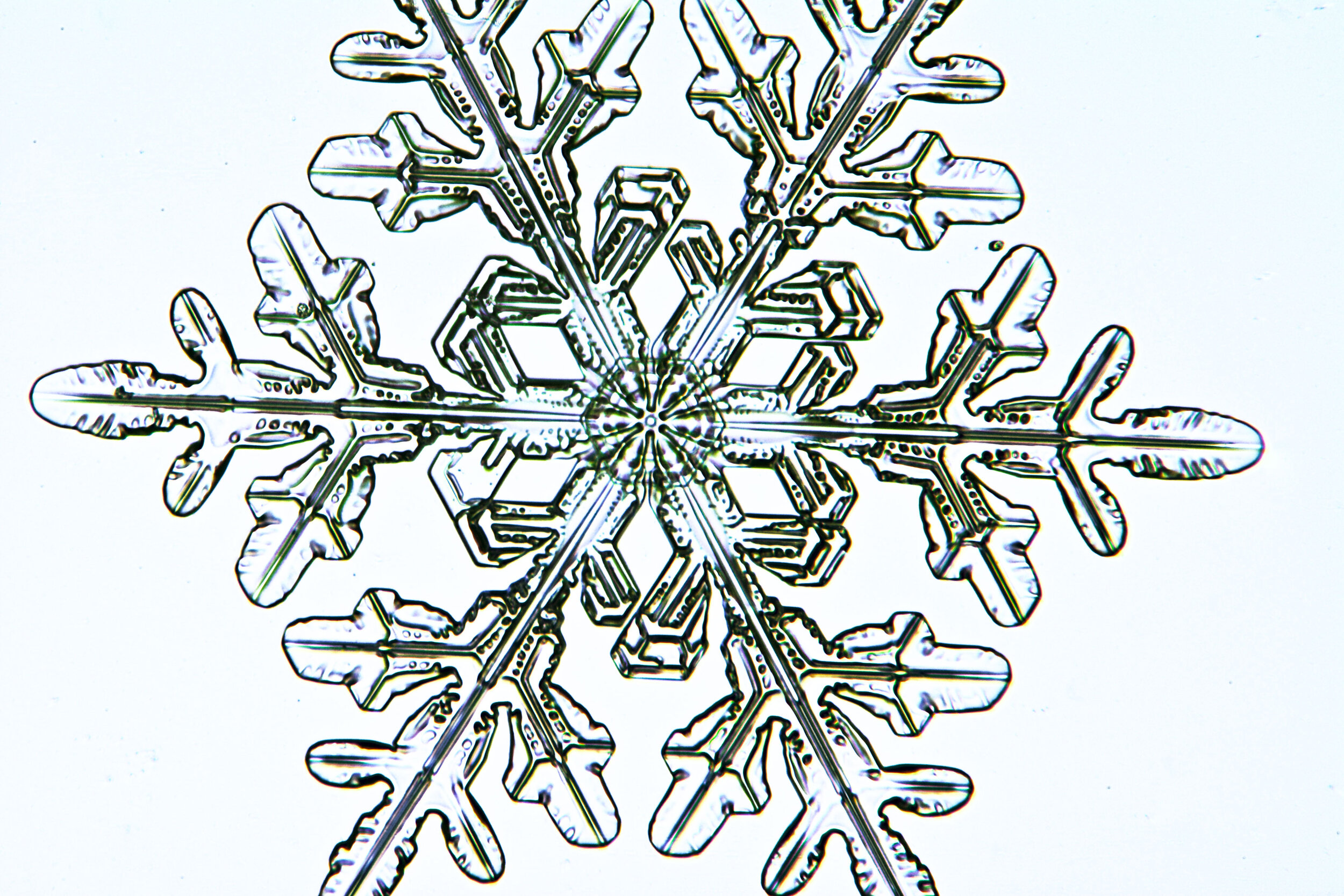



Process Notes:
A good place to observe snow crystals is on the windshield of a car (without the defroster turned on).

STELLAR FORMS:
STELLAR PLATES - STELLAR DENDRITES - TWELVE-SIDED CRYSTALS
Twelve-branch snow crystals
As described above, due to their core crystalline structure, snow crystals are six-sided structures. However, upon occasion, one will encounter 12-sided crystals. These elaborate structures form when two crystals merge as they are forming, and they are often offset such that their branches are evenly distributed.
Plates 6.1 - 6.11

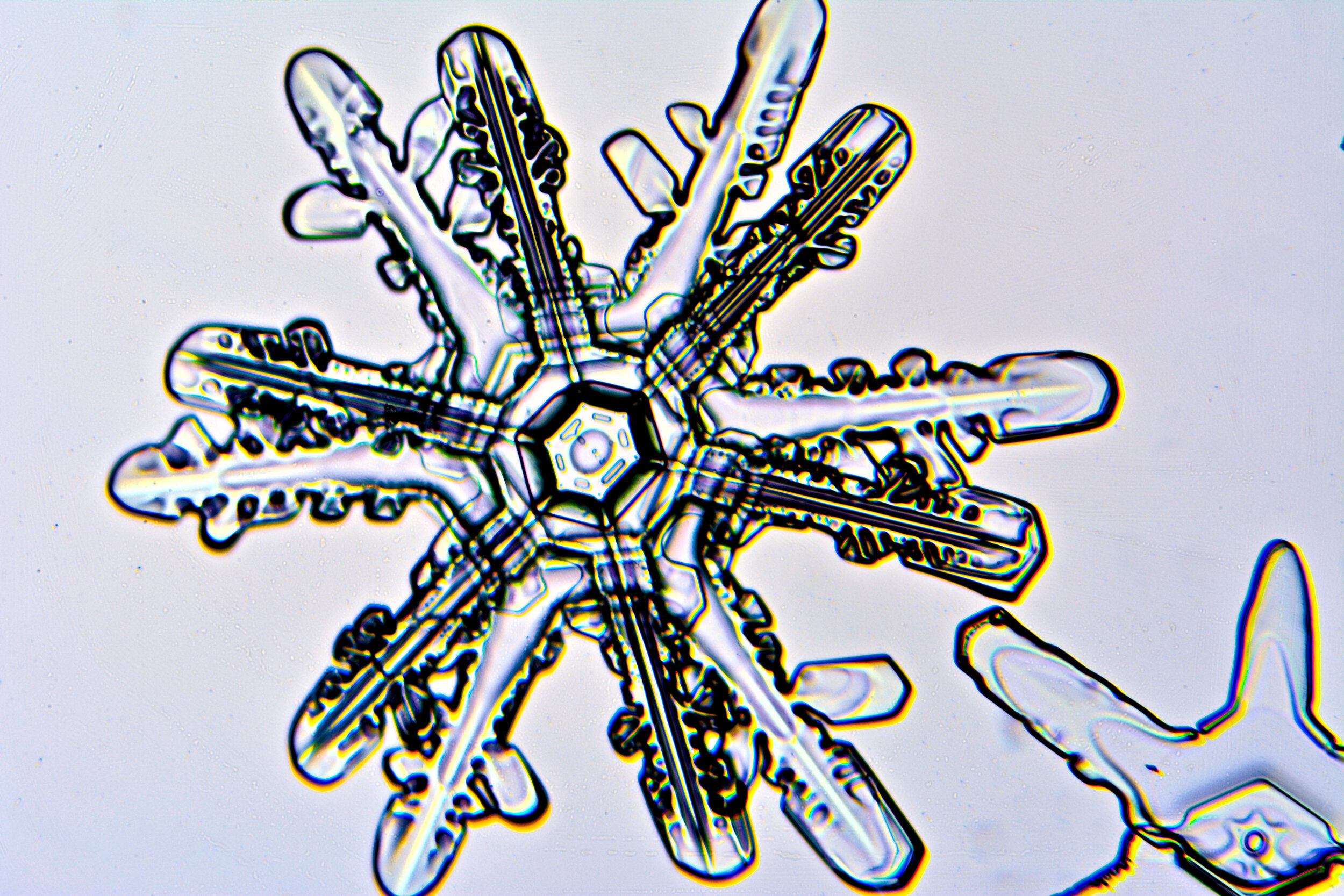
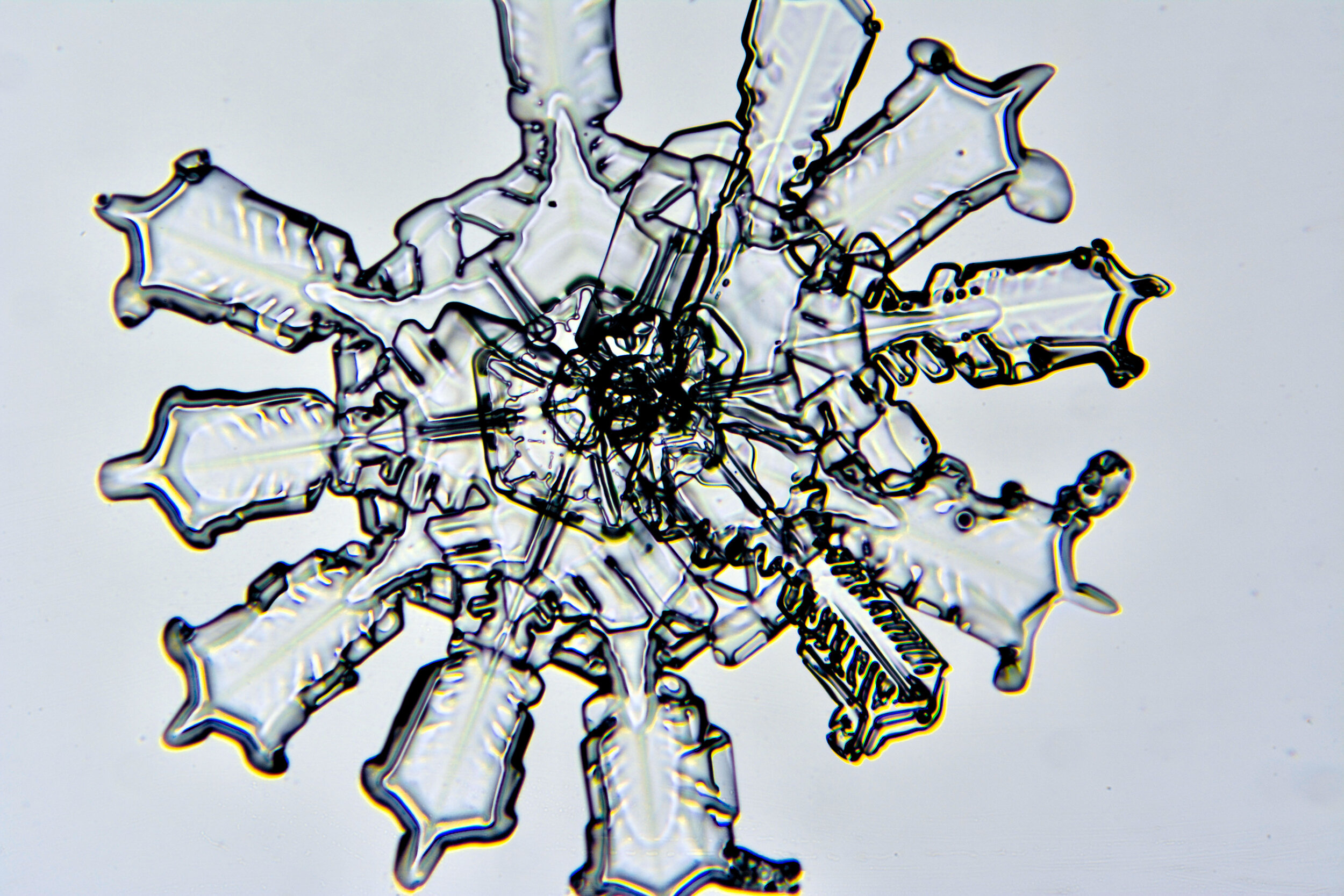




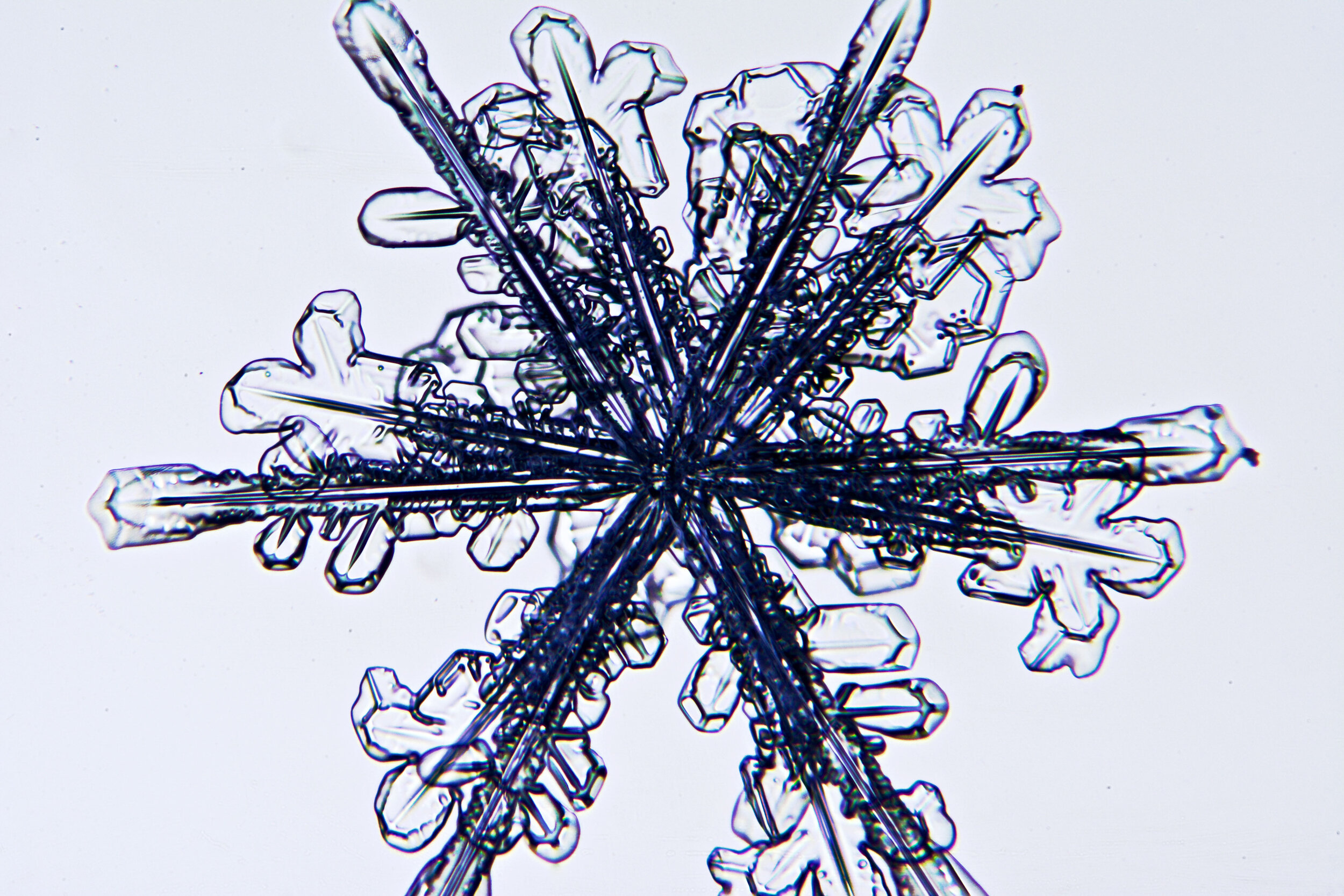



Process Notes:
One of my best sessions was on a crystal-clear morning, about 11°F, when I saw sparkles in the clear blue sky. When I captured some on glass plates, I saw that most of these sparkles were actually perfect snow crystals.


Taking a Closer Look: The Development and Morphology of Snow Crystals
I. LOOK CLOSER - II. CLOSER LOOKS
Acknowledgements & Author Info

Acknowledgments
I would like to acknowledge the inspiration and guidance that I have received by reading the works and admiring the photographs of Wilson “Snowflake” Bentley and Dr. Kenneth Libbrecht.
My snow crystal photography project was inspired by Bentley’s work and guided by the extensive writings of Dr. Libbrecht. Special thanks to Dr. Libbrecht for allowing me to include a schematic diagram from his amazing web site, snowcrystals.com, in this article.
It has been an absolute pleasure working with Stephanie Beattie during the process of organizing and formatting this article. Stephanie’s sense of aesthetics and skills as an editor are evident throughout.

photo by Cheryl Collins
Gary M. Mawe, PhD is the Samuel W. Thayer Professor of Neurological Sciences at the University of Vermont. He and his colleagues conduct research on how the nervous system regulates gastrointestinal function in health and disease, and he teaches a number of courses, including Human Gross Anatomy and Comparative Neurobiology.
Ever since he moved to Vermont in 1988, and learned about the fascinating life of Wilson “Snowflake” Bentley, Mawe has been eager to follow in Bentley’s footsteps and master the art of snow crystal photography. Since December of 2013, he has been welcoming each winter storm as he attempts to capture and photograph pristine snow crystals at his home near the Hinesburg-Huntington border.
Motion is a common theme in Mawe’s artistic endeavors. Two of his favorite photographic subjects (in addition to snow crystals) are waterfalls and fireflies, and he has designed and built a weathervane and a series of mobiles, which he refers to as Mawebiles.



